What Is Considered A Single Electron Group . All of the above (a single bond, a lone pair of electrons, a double bond, and a. The table below indicates the “molecular geometry” of the central atom depending on. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron domains may also be called. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). Electron group geometries refer to the. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Which of the following is considered a single electron group? Which of the following is considered a single electron group? The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry.
from chem.libretexts.org
A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. The table below indicates the “molecular geometry” of the central atom depending on. Which of the following is considered a single electron group? The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. All of the above (a single bond, a lone pair of electrons, a double bond, and a. Which of the following is considered a single electron group?
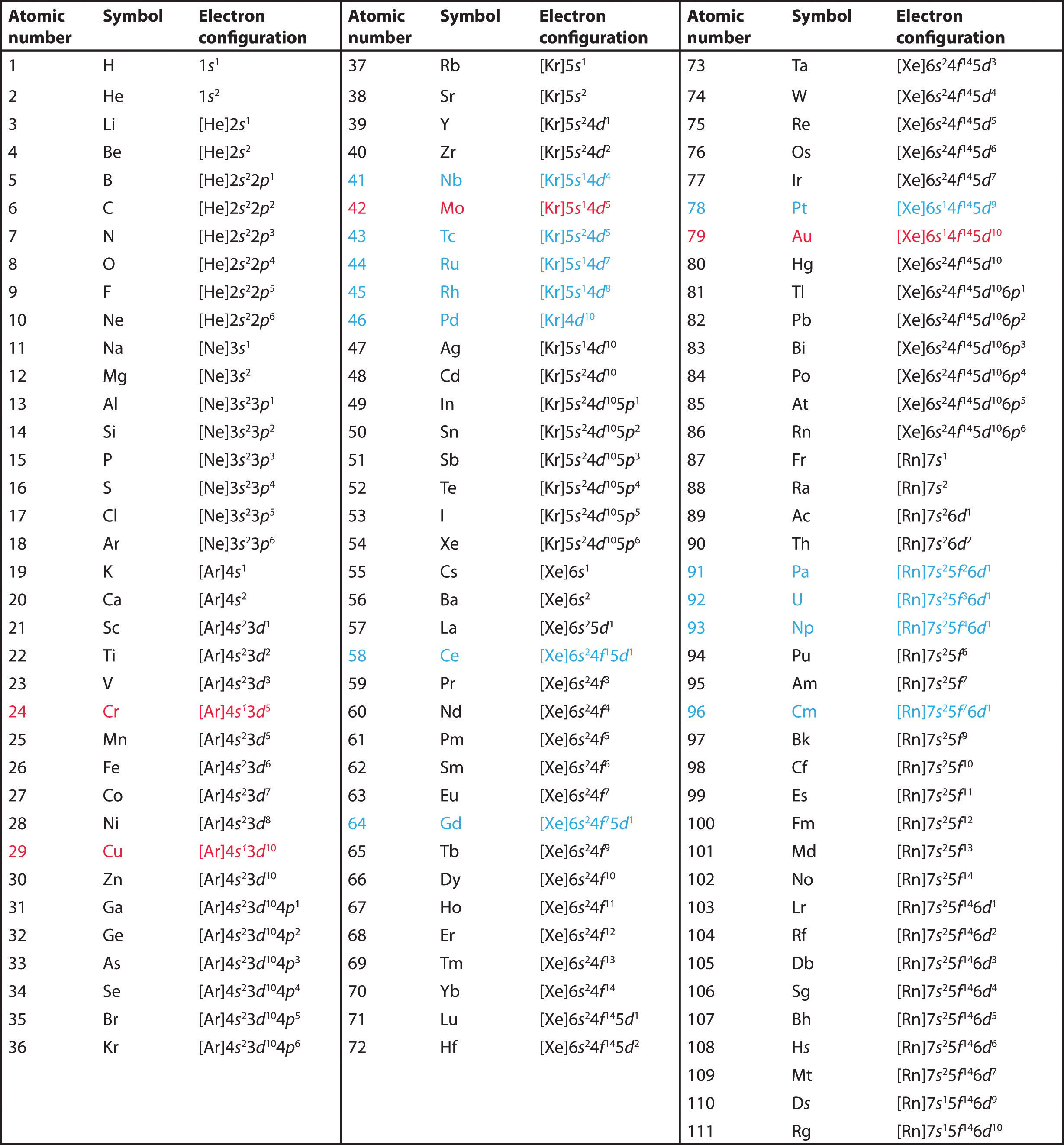
6.9 Electron Configurations & the Periodic Table Chemistry LibreTexts
What Is Considered A Single Electron Group Which of the following is considered a single electron group? All of the above (a single bond, a lone pair of electrons, a double bond, and a. Electron domains may also be called. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. The table below indicates the “molecular geometry” of the central atom depending on. A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). Which of the following is considered a single electron group? Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Which of the following is considered a single electron group? A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Electron group geometries refer to the. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts What Is Considered A Single Electron Group Which of the following is considered a single electron group? Which of the following is considered a single electron group? The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. The table below indicates the “molecular geometry” of the central atom depending on.. What Is Considered A Single Electron Group.
From www.slideserve.com
PPT Molecular Geometry Notes PowerPoint Presentation, free download ID2414914 What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. In chemistry, the electron domain refers to the number of. What Is Considered A Single Electron Group.
From www.ck12.org
The Periodic Table and Electron Configurations CK12 Foundation What Is Considered A Single Electron Group A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Which of the following is considered a single electron group? Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Use figure \(\pageindex{3}\) to determine the molecular geometry. What Is Considered A Single Electron Group.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is Considered A Single Electron Group Electron domains may also be called. The table below indicates the “molecular geometry” of the central atom depending on. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts. What Is Considered A Single Electron Group.
From study.com
Predicting the Arrangement of Electron Groups Around the Central Atom of a Molecule Chemistry What Is Considered A Single Electron Group The table below indicates the “molecular geometry” of the central atom depending on. All of the above (a single bond, a lone pair of electrons, a double bond, and a. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. The number of bonds to the central atom. What Is Considered A Single Electron Group.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Which of the following is considered a single electron group? Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group.. What Is Considered A Single Electron Group.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry What Is Considered A Single Electron Group All of the above (a single bond, a lone pair of electrons, a double bond, and a. Electron group geometries refer to the. A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond. What Is Considered A Single Electron Group.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is Considered A Single Electron Group A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). Electron domains may also be called. The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. A “group” of electrons can be a single. What Is Considered A Single Electron Group.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Is Considered A Single Electron Group Which of the following is considered a single electron group? Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. A “group” of electrons can. What Is Considered A Single Electron Group.
From fesschange.weebly.com
Electron geometry chart 8 electron groups fesschange What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. All of the above (a single bond, a lone pair. What Is Considered A Single Electron Group.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind the Atoms Together What Is Considered A Single Electron Group The table below indicates the “molecular geometry” of the central atom depending on. The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. Which of the following is considered a single. What Is Considered A Single Electron Group.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica What Is Considered A Single Electron Group All of the above (a single bond, a lone pair of electrons, a double bond, and a. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Which of the following is considered a single electron group? In chemistry, the electron domain refers to the number of. What Is Considered A Single Electron Group.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table What Is Considered A Single Electron Group In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron group geometries refer to the. The table below indicates the “molecular geometry” of the central atom depending on. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. The number of bonds to the central. What Is Considered A Single Electron Group.
From studylib.net
Electron Groups on Central Atom What Is Considered A Single Electron Group Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Electron domains may also be called. Which of the following is considered a single electron group? A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Which of. What Is Considered A Single Electron Group.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Chemistry 103/104 Resource Book What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Electron domains may also be called. A “group” of electrons can. What Is Considered A Single Electron Group.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps What Is Considered A Single Electron Group A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Which of the following is considered a single electron group? In chemistry, the electron domain refers to the. What Is Considered A Single Electron Group.
From www.britannica.com
Periodic table Elements, Groups, Properties Britannica What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Which of the following is considered a single electron group? Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. A “group” of electrons can be a single bond, double bond,. What Is Considered A Single Electron Group.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram What Is Considered A Single Electron Group Which of the following is considered a single electron group? Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. The table below indicates the “molecular geometry” of the central atom depending on. Electron domains may also be called. In chemistry, the electron domain refers to the. What Is Considered A Single Electron Group.
From cartridges.esciencelabs.com
Diagram of shapes of molecules, showng bonding pairs, arrangement of electron domains, electron What Is Considered A Single Electron Group Electron group geometries refer to the. Which of the following is considered a single electron group? Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Which of the following is considered a single electron group? A) a lone pair of electrons b) a single bond c). What Is Considered A Single Electron Group.
From studylib.net
One Page Lesson Determining Electron What Is Considered A Single Electron Group A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond. What Is Considered A Single Electron Group.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules What Is Considered A Single Electron Group Electron domains may also be called. Which of the following is considered a single electron group? In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is. What Is Considered A Single Electron Group.
From www.slideserve.com
PPT Chapter 10 Chemical Bonding II PowerPoint Presentation, free download ID3509787 What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Which of the following is considered a single electron group?. What Is Considered A Single Electron Group.
From sciencenotes.org
List of Electron Configurations of Elements What Is Considered A Single Electron Group All of the above (a single bond, a lone pair of electrons, a double bond, and a. Which of the following is considered a single electron group? A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Electron group geometries refer to the. Count the number of electron groups around each. What Is Considered A Single Electron Group.
From courses.lumenlearning.com
3.4 Electronic Structure of Atoms (Electron Configurations) General College Chemistry I What Is Considered A Single Electron Group A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). Which of the following is considered a single electron group?. What Is Considered A Single Electron Group.
From homedeso.vercel.app
Group 1 Periodic Table Electron Configuration What Is Considered A Single Electron Group Which of the following is considered a single electron group? In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). The number of bonds to the central atom plus. What Is Considered A Single Electron Group.
From www.slideserve.com
PPT Molecular Geometry Notes PowerPoint Presentation, free download ID2414914 What Is Considered A Single Electron Group Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). All of the above (a single bond, a lone pair of electrons, a double bond, and a. Which. What Is Considered A Single Electron Group.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules What Is Considered A Single Electron Group In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. All of the above (a single bond, a lone pair of electrons, a double bond, and a. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a. What Is Considered A Single Electron Group.
From www.britannica.com
Chemical compound Trends, Elements, Properties Britannica What Is Considered A Single Electron Group The table below indicates the “molecular geometry” of the central atom depending on. Electron group geometries refer to the. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Electron domains may also be called. In chemistry, the electron domain refers to the number of lone pairs. What Is Considered A Single Electron Group.
From courses.lumenlearning.com
Electrons Biology for Majors I What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Which of the following is considered a single electron group? In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. All. What Is Considered A Single Electron Group.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Considered A Single Electron Group Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Electron domains may also be called. Electron group geometries refer to the. In chemistry,. What Is Considered A Single Electron Group.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Trends ALevel What Is Considered A Single Electron Group Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. A) a lone pair of electrons b) a single bond c) a double bond d) a triple bond e). Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond. What Is Considered A Single Electron Group.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Considered A Single Electron Group Electron group geometries refer to the. In chemistry, the electron domain refers to the number of lone pairs or bond locations around a particular atom in a molecule. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. Which of the following is considered a single electron group? Count the number of electron groups around each carbon, recognizing. What Is Considered A Single Electron Group.
From www.chemistrysteps.com
VSEPR Theory Geometry of Organic Molecules Chemistry Steps What Is Considered A Single Electron Group The table below indicates the “molecular geometry” of the central atom depending on. A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. Use figure \(\pageindex{3}\) to determine the molecular geometry around each carbon atom. In chemistry, the electron domain refers to the number of lone pairs or bond locations around. What Is Considered A Single Electron Group.
From chem.libretexts.org
6.9 Electron Configurations & the Periodic Table Chemistry LibreTexts What Is Considered A Single Electron Group The number of bonds to the central atom plus the number of lone pairs on the central atom gives us what is called the electron group geometry. Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. Electron group geometries refer to the. Which of the following. What Is Considered A Single Electron Group.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Is Considered A Single Electron Group Count the number of electron groups around each carbon, recognizing that in the vsepr model, a multiple bond counts as a single group. A “group” of electrons can be a single bond, double bond, triple bond, or a lone pair of electrons. The table below indicates the “molecular geometry” of the central atom depending on. A) a lone pair of. What Is Considered A Single Electron Group.