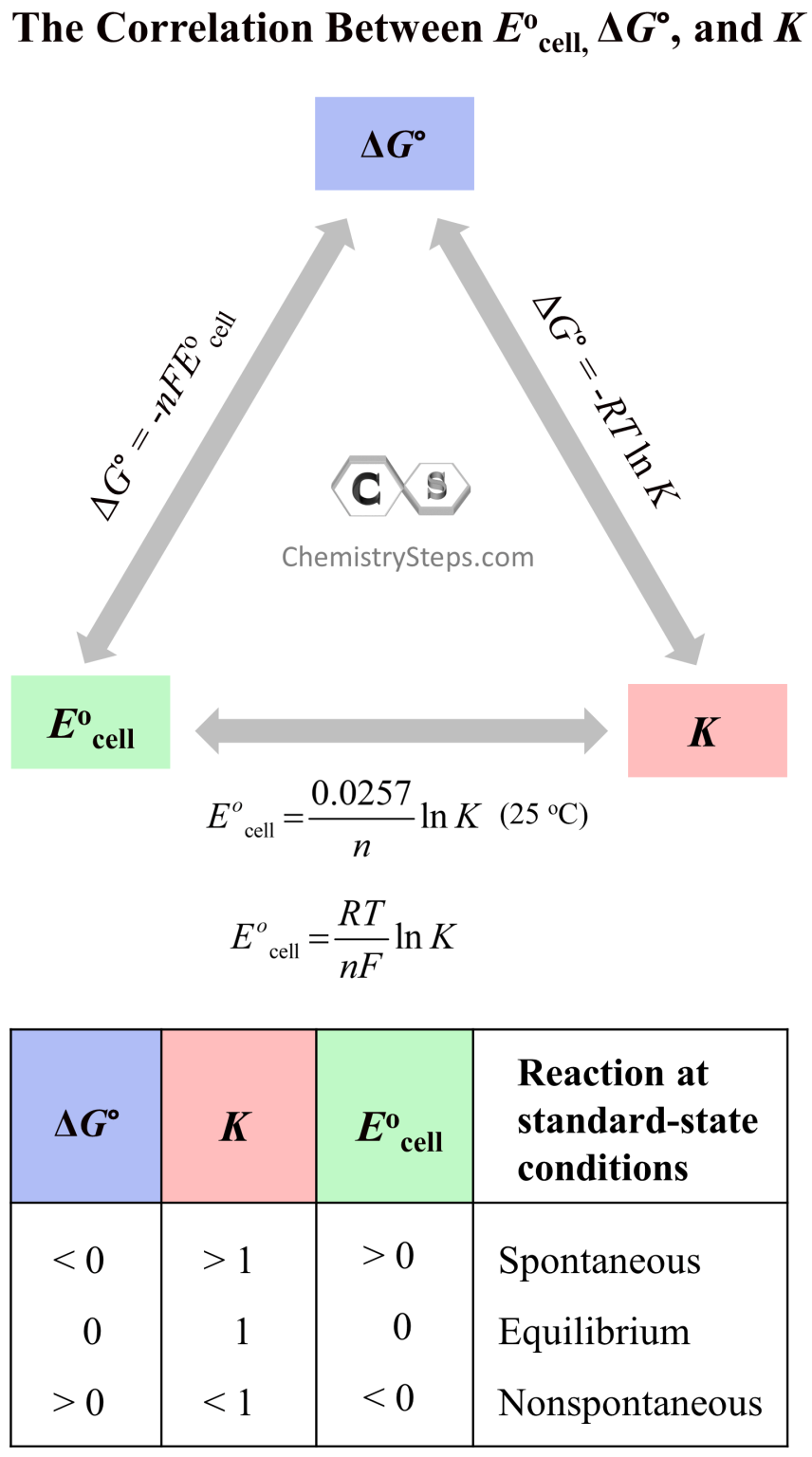
Standard Cell Potential For A Spontaneous Reaction . The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. From this value, determine whether the overall reaction is spontaneous. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. Unlike the spontaneous oxidation of copper by. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. Unlike the spontaneous oxidation of copper by.
from general.chemistrysteps.com
A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Unlike the spontaneous oxidation of copper by. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. From this value, determine whether the overall reaction is spontaneous. Unlike the spontaneous oxidation of copper by. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes.
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps
Standard Cell Potential For A Spontaneous Reaction A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: From this value, determine whether the overall reaction is spontaneous. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. Unlike the spontaneous oxidation of copper by. Unlike the spontaneous oxidation of copper by. Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called.
From www.numerade.com
SOLVED What is the standard cell potential for the spontaneous voltaic Standard Cell Potential For A Spontaneous Reaction Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Describe and relate the definitions of electrode and cell potentials. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. The standard cell potential (e°cell) is measured at 298. Standard Cell Potential For A Spontaneous Reaction.
From www.chegg.com
Solved Calculate the standard cell potential for the Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Unlike the spontaneous oxidation of copper by. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. The potential of. Standard Cell Potential For A Spontaneous Reaction.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Standard Cell Potential For A Spontaneous Reaction Calculate cell potentials and predict redox spontaneity using standard electrode potentials. From this value, determine whether the overall reaction is spontaneous. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Unlike the spontaneous oxidation. Standard Cell Potential For A Spontaneous Reaction.
From www.chegg.com
Solved 5. Calculate the standard cell potential for the Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. Unlike the spontaneous oxidation of copper by. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at. Standard Cell Potential For A Spontaneous Reaction.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential For A Spontaneous Reaction A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Unlike the spontaneous oxidation of copper by. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. From this value,. Standard Cell Potential For A Spontaneous Reaction.
From toppr.com
Consider the following electrochemical cell1.Write a balanced net Standard Cell Potential For A Spontaneous Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. From this value, determine whether the overall reaction is spontaneous. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. Calculate cell potentials and predict redox spontaneity using standard. Standard Cell Potential For A Spontaneous Reaction.
From www.chegg.com
Solved Calculate spontaneous standard cell potentials (Ece) Standard Cell Potential For A Spontaneous Reaction A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Calculate cell potentials and predict redox spontaneity using standard electrode. Standard Cell Potential For A Spontaneous Reaction.
From www.bartleby.com
Answered Given the following two halfreactions,… bartleby Standard Cell Potential For A Spontaneous Reaction A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Unlike the spontaneous oxidation of copper by. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. From this value, determine whether the overall reaction is spontaneous. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and. Standard Cell Potential For A Spontaneous Reaction.
From www.expii.com
Spontaneous and Nonspontaneous Reactions — Overview Expii Standard Cell Potential For A Spontaneous Reaction Interpret electrode potentials in terms of relative oxidant and reductant strengths. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: From this value, determine whether the overall reaction is spontaneous. 1 atm for gases, 1 m for solutions, and the pure. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVED Calculate the standard cell potential for the following Standard Cell Potential For A Spontaneous Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Describe and relate the. Standard Cell Potential For A Spontaneous Reaction.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential For A Spontaneous Reaction Describe and relate the definitions of electrode and cell potentials. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. From this value, determine whether the overall reaction is spontaneous. Calculate cell potentials and predict redox spontaneity using. Standard Cell Potential For A Spontaneous Reaction.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Use equation \(\ref{19.10}\) to calculate the standard cell potential for. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVED Use tabulated standard electrode potentials to calculate the Standard Cell Potential For A Spontaneous Reaction The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Calculate cell potentials and predict redox spontaneity using standard electrode potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Unlike the spontaneous oxidation of copper by. Describe and relate the definitions of electrode and cell potentials. The. Standard Cell Potential For A Spontaneous Reaction.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 Standard Cell Potential For A Spontaneous Reaction Describe and relate the definitions of electrode and cell potentials. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. Unlike the spontaneous oxidation of copper by. Unlike the spontaneous oxidation of copper by. From this value, determine whether the overall reaction is spontaneous. A redox reaction is spontaneous if the standard electrode potential for the redox. Standard Cell Potential For A Spontaneous Reaction.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Describe and relate the definitions of electrode and cell potentials. Unlike the spontaneous oxidation of copper by. The standard cell potential (e°cell) is. Standard Cell Potential For A Spontaneous Reaction.
From www.chegg.com
Solved For each reaction listed, determine its standard cell Standard Cell Potential For A Spontaneous Reaction 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Unlike the spontaneous oxidation of copper by. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVEDHow are standard reduction potentials combined to give the Standard Cell Potential For A Spontaneous Reaction The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Unlike the spontaneous oxidation of copper by. Describe and relate the definitions of electrode and cell potentials. From this value, determine whether the overall reaction is spontaneous. A redox. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVED Based on the sign of the standard cell potential. Eau Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Describe and relate the definitions of electrode and cell potentials. Unlike the spontaneous oxidation of copper by. Calculate cell potentials and. Standard Cell Potential For A Spontaneous Reaction.
From www.chegg.com
Solved For each reaction listed, determine its standard cell Standard Cell Potential For A Spontaneous Reaction Describe and relate the definitions of electrode and cell potentials. From this value, determine whether the overall reaction is spontaneous. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Unlike the spontaneous oxidation of copper by. Use equation \(\ref{19.10}\). Standard Cell Potential For A Spontaneous Reaction.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential For A Spontaneous Reaction The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. From this value, determine whether the overall reaction is spontaneous. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Interpret electrode potentials. Standard Cell Potential For A Spontaneous Reaction.
From www.slideserve.com
PPT Cell Potential and Nernst Equation PowerPoint Presentation, free Standard Cell Potential For A Spontaneous Reaction The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Unlike. Standard Cell Potential For A Spontaneous Reaction.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential For A Spontaneous Reaction Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. From this value, determine whether the overall reaction is spontaneous. Unlike the spontaneous oxidation of copper by. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or. Standard Cell Potential For A Spontaneous Reaction.
From www.slideserve.com
PPT Electrochemistry Part IV Spontaneity & Nernst Equation Standard Cell Potential For A Spontaneous Reaction A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. Unlike the spontaneous. Standard Cell Potential For A Spontaneous Reaction.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: From this value, determine whether the overall reaction is spontaneous. The. Standard Cell Potential For A Spontaneous Reaction.
From www.chegg.com
Solved 4. Calculate the standard cell potential for each Standard Cell Potential For A Spontaneous Reaction Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Calculate cell potentials and predict redox spontaneity using. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVED Calculate the cell potential, E for the given reactions at 25. Standard Cell Potential For A Spontaneous Reaction Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Unlike the spontaneous oxidation of copper by. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. Unlike. Standard Cell Potential For A Spontaneous Reaction.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Standard Cell Potential For A Spontaneous Reaction Calculate cell potentials and predict redox spontaneity using standard electrode potentials. From this value, determine whether the overall reaction is spontaneous. Describe and relate the definitions of electrode and cell potentials. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids. Standard Cell Potential For A Spontaneous Reaction.
From www.toppr.com
The reaction is spontaneous the cell potential is Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes.. Standard Cell Potential For A Spontaneous Reaction.
From www.slideserve.com
PPT 14.2b Standard Cells and Cell Potential PowerPoint Presentation Standard Cell Potential For A Spontaneous Reaction The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Unlike the spontaneous oxidation of copper by. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. Describe and relate the. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVED 1 Calculate the standard cell potential (from the Standard Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. The standard cell potential (e°cell). Standard Cell Potential For A Spontaneous Reaction.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. From this value, determine whether the overall reaction is spontaneous. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Unlike. Standard Cell Potential For A Spontaneous Reaction.
From www.youtube.com
Electrochem 02 Using Standard Cell Potential to Determine Spontaneity Standard Cell Potential For A Spontaneous Reaction Unlike the spontaneous oxidation of copper by. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. A redox reaction is spontaneous if the standard electrode potential for the redox reaction, e o(redox reaction), is positive. Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 atm for gases, 1 m for solutions, and the pure solid. Standard Cell Potential For A Spontaneous Reaction.
From www.chegg.com
Solved Based on the sign of the standard cell potential, Standard Cell Potential For A Spontaneous Reaction The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Unlike the spontaneous oxidation of copper by. Unlike the spontaneous oxidation of copper by. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVED 14. [20Q36,37,38] The standard Gibbs free energy (ΔG) of the Standard Cell Potential For A Spontaneous Reaction Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Unlike the spontaneous oxidation of copper by. Calculate cell potentials and predict redox spontaneity using standard electrode potentials. Use equation \(\ref{19.10}\) to calculate the standard cell potential for the overall reaction. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1. Standard Cell Potential For A Spontaneous Reaction.
From www.numerade.com
SOLVED What is the standard cell potential for the spontaneous Standard Cell Potential For A Spontaneous Reaction The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Unlike the spontaneous oxidation of copper by. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. A redox reaction is spontaneous. Standard Cell Potential For A Spontaneous Reaction.