Partition Meaning Chemistry . Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. Solvent partitioning requires two solvents that are not miscible in each other. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). Usually one of the solvents is water. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a.
from www.eng.buffalo.edu
The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. Solvent partitioning requires two solvents that are not miscible in each other. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. Usually one of the solvents is water. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. A partition coefficient is the ratio of a solute in two different solvents.
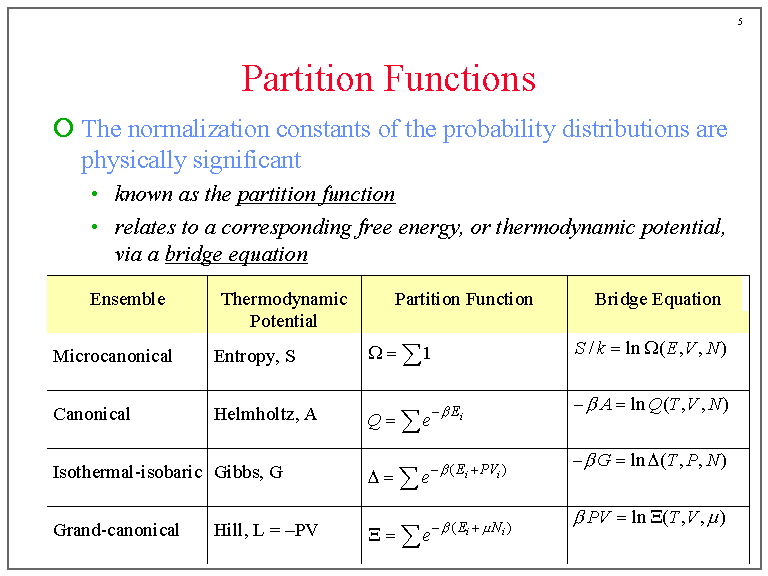
Partition Functions
Partition Meaning Chemistry Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Solvent partitioning requires two solvents that are not miscible in each other. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Usually one of the solvents is water. A partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases).
From www.youtube.com
(Abstract Algebra 1) Definition of a Partition YouTube Partition Meaning Chemistry The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Solvent partitioning requires two solvents that are not miscible in each other. How is the partition function of the system built up from those of the subsystems depends on. Partition Meaning Chemistry.
From pubs.acs.org
Calculating Partition Coefficients of Small Molecules in Octanol/Water Partition Meaning Chemistry A partition coefficient is the ratio of a solute in two different solvents. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). How is the partition function of the system built. Partition Meaning Chemistry.
From www.youtube.com
Solubility Partition Coefficient physicochemical properties (P2 Partition Meaning Chemistry Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. The partition coefficient. Partition Meaning Chemistry.
From www.studypool.com
SOLUTION Physical chemistry boltzmann factor and partition functions Partition Meaning Chemistry Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. A partition coefficient is the ratio of a solute in two different solvents. The partition or sorption coefficient k may be expressed as the sum of five interaction. Partition Meaning Chemistry.
From www.mdpi.com
Entropy Free FullText Graph Partitions in Chemistry Partition Meaning Chemistry Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. How is the partition function of. Partition Meaning Chemistry.
From www.slideserve.com
PPT Partition Coefficients PowerPoint Presentation, free download Partition Meaning Chemistry Usually one of the solvents is water. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The ratio. Partition Meaning Chemistry.
From pubs.acs.org
OctanolWater Partition Coefficient Measurement by a Simple 1H NMR Partition Meaning Chemistry Solvent partitioning requires two solvents that are not miscible in each other. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. Usually one of the solvents is water. The partition coefficient (k pc) is the ratio of the concentrations of. Partition Meaning Chemistry.
From www.sliderbase.com
Vapor Pressure of Solutions Presentation Chemistry Partition Meaning Chemistry The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous. Partition Meaning Chemistry.
From www.lifewire.com
What Is a Partition? (Disk Partition Definition) Partition Meaning Chemistry Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). Usually one of the solvents is water. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to. Partition Meaning Chemistry.
From www.docsity.com
Partition PhysicsLecture Slides Docsity Partition Meaning Chemistry A partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). Solvent partitioning requires two solvents that are not miscible in each other. The. Partition Meaning Chemistry.
From www.youtube.com
What are Periods and Groups in the Periodic Table Chemistry The Fuse Partition Meaning Chemistry Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. A partition coefficient. Partition Meaning Chemistry.
From exyyyxhal.blob.core.windows.net
Partition Definition Structure at Walter Werner blog Partition Meaning Chemistry Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact. Partition Meaning Chemistry.
From www.chegg.com
Solved 4. The grand partition function In the grand Partition Meaning Chemistry Usually one of the solvents is water. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). Solvent partitioning requires two solvents that are not miscible in each other. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each. Partition Meaning Chemistry.
From www.youtube.com
partition chromatography YouTube Partition Meaning Chemistry The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). The ratio of a compound’s unionized species concentrations in a mixture. Partition Meaning Chemistry.
From www.researchgate.net
(PDF) Graph Partitions in Chemistry Partition Meaning Chemistry Usually one of the solvents is water. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Solvent partitioning requires two solvents that are not. Partition Meaning Chemistry.
From ceziulkb.blob.core.windows.net
How Many Type Of Partition Chromatography at Gwendolyn Rucker blog Partition Meaning Chemistry The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. Usually one of the solvents is water. Solvent partitioning requires two solvents that are not miscible in each other. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact. Partition Meaning Chemistry.
From www.slideserve.com
PPT PARTITION FUNCTION PowerPoint Presentation, free download ID Partition Meaning Chemistry The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. A partition coefficient is the ratio of a solute. Partition Meaning Chemistry.
From www.slideserve.com
PPT Liquid Chromatography PowerPoint Presentation, free download ID Partition Meaning Chemistry Usually one of the solvents is water. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. A partition coefficient is the ratio of a solute in two different solvents. How is the partition function of the system built. Partition Meaning Chemistry.
From www.eng.buffalo.edu
Partition Functions Partition Meaning Chemistry The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. The partition or sorption coefficient k may. Partition Meaning Chemistry.
From www.slideserve.com
PPT Lipinski’s rule of five PowerPoint Presentation, free download Partition Meaning Chemistry Usually one of the solvents is water. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. The partition or sorption coefficient k may be expressed as the sum of five interaction terms,. Partition Meaning Chemistry.
From microbenotes.com
Paper Chromatography Definition, Types, Principle, Steps, Uses Partition Meaning Chemistry How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. Usually one of the solvents is water. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. Partitioning is the distribution of a solute, s, between two. Partition Meaning Chemistry.
From www.youtube.com
The Canonical Partition Function YouTube Partition Meaning Chemistry Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. Usually one of the solvents is water. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. How is the partition function of the system built up. Partition Meaning Chemistry.
From www.slideshare.net
Partition coefficient PPT Partition Meaning Chemistry Solvent partitioning requires two solvents that are not miscible in each other. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. How is the partition function of the system built up from those of the. Partition Meaning Chemistry.
From www.chegg.com
Solved In Chromatography, The Partition Coefficient, K, D... Partition Meaning Chemistry The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). Usually one of the solvents is water. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. Solvent partitioning requires two solvents. Partition Meaning Chemistry.
From www.youtube.com
Partition Coefficient Organic Chemistry Lab lecture YouTube Partition Meaning Chemistry The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Usually one of the solvents is water. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable. Partition Meaning Chemistry.
From www.youtube.com
The Molecular Partition Function YouTube Partition Meaning Chemistry Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). Usually one of the solvents is water. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. How is the partition. Partition Meaning Chemistry.
From www.youtube.com
Partition Meaning YouTube Partition Meaning Chemistry The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. Usually one of the solvents is water. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate.. Partition Meaning Chemistry.
From www.hanlin.com
CIE A Level Chemistry复习笔记5.5.9 Partition Coefficients翰林国际教育 Partition Meaning Chemistry The ratio of a compound’s unionized species concentrations in a mixture of two immiscible. A partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with. Partition Meaning Chemistry.
From themasterchemistry.com
Distribution Law In ChemistryDefinition, Explanation, And Applications Partition Meaning Chemistry Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a.. Partition Meaning Chemistry.
From www.numerade.com
SOLVED For the compounds HCI and SO2, with the element information Partition Meaning Chemistry The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. Solubility (grams per 100 ml) or. Partition Meaning Chemistry.
From www.slideserve.com
PPT Contaminant Hydrogeology III PowerPoint Presentation, free Partition Meaning Chemistry A partition coefficient is the ratio of a solute in two different solvents. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate.. Partition Meaning Chemistry.
From www.youtube.com
StatMolThermo 09.07 Chemical Potential from the Partition Function Partition Meaning Chemistry How is the partition function of the system built up from those of the subsystems depends on whether the subsystems are distinguishable or. A partition coefficient is the ratio of a solute in two different solvents. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. Usually one of the solvents is water. The. Partition Meaning Chemistry.
From www.slideserve.com
PPT PARTITION FUNCTION PowerPoint Presentation, free download ID Partition Meaning Chemistry A partition coefficient is the ratio of a solute in two different solvents. Solvent partitioning requires two solvents that are not miscible in each other. The partition or sorption coefficient k may be expressed as the sum of five interaction terms, with the uppercase parameters. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to. Partition Meaning Chemistry.
From www.youtube.com
Partition Function Introduction and significance YouTube Partition Meaning Chemistry Partitioning is the distribution of a solute, s, between two immiscible solvents (such as aqueous and organic phases). The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The partition or sorption coefficient k may be expressed as the. Partition Meaning Chemistry.
From www.slideserve.com
PPT Reaction Rate Theory PowerPoint Presentation, free download ID Partition Meaning Chemistry Solvent partitioning requires two solvents that are not miscible in each other. Solubility (grams per 100 ml) or molarity concentrations (mole per liter) are often used to evlauate. The partition coefficient (k pc) is the ratio of the concentrations of a solute in two different immiscible solvents in contact with each other when equilibrium has been established (at a. The. Partition Meaning Chemistry.