How Do You Solve Dilution Problems . — i'll show you how to solve some common dilution problems using the. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Understand how stock solutions are used in the laboratory. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. explain how concentrations can be changed in the lab. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using.
from www.youtube.com
Of course, the resulting solution is. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. to dilute a solution means to add more solvent without the addition of more solute. — i'll show you how to solve some common dilution problems using the. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without.
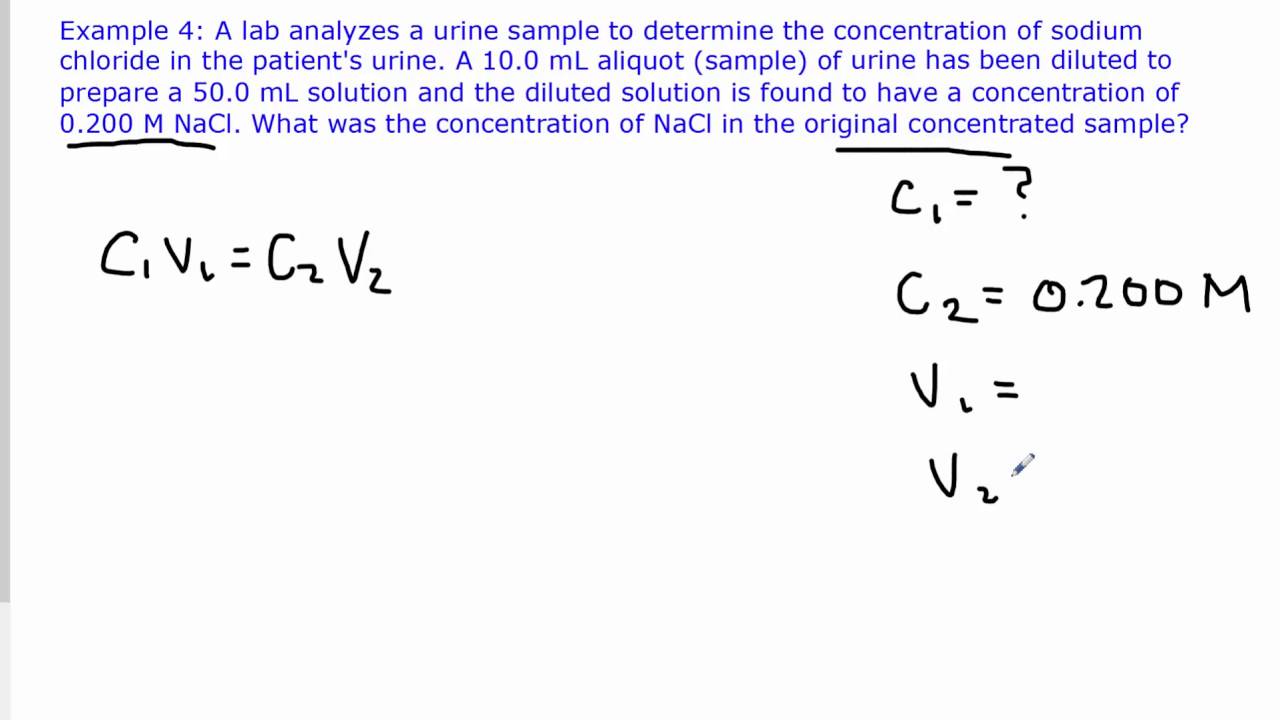
Chemistry 11 Dilution Calculations Solving for Initial Concentration
How Do You Solve Dilution Problems Of course, the resulting solution is. — i'll show you how to solve some common dilution problems using the. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. to dilute a solution means to add more solvent without the addition of more solute. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. Understand how stock solutions are used in the laboratory. Of course, the resulting solution is.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube How Do You Solve Dilution Problems — i'll show you how to solve some common dilution problems using the. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. Of course, the resulting solution is. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. to. How Do You Solve Dilution Problems.
From www.scribd.com
dilution tutorial and problems Significant Figures Concentration How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. explain how concentrations can be changed in the lab. Of course, the resulting solution is. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. Understand how stock solutions are used in the laboratory. — i'll show you how to solve some common dilution problems using the. — this is a chemistry tutorial that covers dilution problems,. How Do You Solve Dilution Problems.
From www.tffn.net
Solving Dilution Problems A StepbyStep Guide The Enlightened Mindset How Do You Solve Dilution Problems explain how concentrations can be changed in the lab. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — for example, calculate the. How Do You Solve Dilution Problems.
From www.tffn.net
Solving Dilution Problems A StepbyStep Guide The Enlightened Mindset How Do You Solve Dilution Problems explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new.. How Do You Solve Dilution Problems.
From www.chegg.com
Solved Dilution Problems For problems 7 11 use the dilution How Do You Solve Dilution Problems — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Understand how stock solutions are used in the laboratory. Of course, the resulting solution is. explain how concentrations can be changed in the lab. — for example, calculate the volume of a 99.5% v/v solution of. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube How Do You Solve Dilution Problems to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3. How Do You Solve Dilution Problems.
From www.slideshare.net
Molarity and dilution How Do You Solve Dilution Problems Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. to dilute a solution means to add more solvent without the addition of more solute. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — i'll. How Do You Solve Dilution Problems.
From www.pinterest.com
DILUTIONS M1V1; M2V2 Solving Dilution Problems in Solution Chemistry How Do You Solve Dilution Problems — i'll show you how to solve some common dilution problems using the. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. Of course, the resulting solution is. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the. How Do You Solve Dilution Problems.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration How Do You Solve Dilution Problems — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. to dilute a solution means to add more solvent without the addition of more solute.. How Do You Solve Dilution Problems.
From www.youtube.com
How solve dilution and concentration calculation problems YouTube How Do You Solve Dilution Problems Understand how stock solutions are used in the laboratory. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. explain how concentrations can be changed in the lab. Of course, the resulting. How Do You Solve Dilution Problems.
From www.slideshare.net
Molarity Molality Dilutions How Do You Solve Dilution Problems — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. Of course, the resulting solution is. — a dilution is a process where the concentration of a solution is lowered by adding. How Do You Solve Dilution Problems.
From dxopdbmbn.blob.core.windows.net
Dilution Symbol at Andrea Bloom blog How Do You Solve Dilution Problems Of course, the resulting solution is. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — for example, calculate the volume of a 99.5% v/v solution of. How Do You Solve Dilution Problems.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID How Do You Solve Dilution Problems — i'll show you how to solve some common dilution problems using the. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be. How Do You Solve Dilution Problems.
From www.youtube.com
Dilutions Explained with Problems YouTube How Do You Solve Dilution Problems — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. to dilute a solution means to add more solvent without the addition of more solute. — i'll show you how to solve some common dilution problems. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate How Do You Solve Dilution Problems Of course, the resulting solution is. to dilute a solution means to add more solvent without the addition of more solute. — i'll show you how to solve some common dilution problems using the. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. — a dilution is. How Do You Solve Dilution Problems.
From www.youtube.com
How to solve dilution problems? كيفية عمل الحسابات المتعلقة بتخفيف How Do You Solve Dilution Problems — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. — i'll show you how to solve some common dilution problems using the. Understand how. How Do You Solve Dilution Problems.
From www.youtube.com
Molarity Dilution Practice Problem 3 YouTube How Do You Solve Dilution Problems — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. Of course, the resulting solution is. Understand how stock solutions are used in the laboratory. to dilute a solution. How Do You Solve Dilution Problems.
From www.youtube.com
Molarity Dilution Practice Problem 2 YouTube How Do You Solve Dilution Problems — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. Understand how stock solutions are used in the laboratory. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. — for example, calculate the volume of a 99.5% v/v solution of acetic. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution Calculation Practice YouTube How Do You Solve Dilution Problems — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. to dilute a solution means to add more solvent without the addition of more solute. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. Of course, the resulting. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. — a dilution is a process where the concentration of a. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube How Do You Solve Dilution Problems to dilute a solution means to add more solvent without the addition of more solute. Understand how stock solutions are used in the laboratory. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. — this chemistry video tutorial explains how to. How Do You Solve Dilution Problems.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. Of course, the resulting solution is. Understand how stock solutions are used in the laboratory.. How Do You Solve Dilution Problems.
From www.youtube.com
Solving dilution problems YouTube How Do You Solve Dilution Problems — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. Understand how stock solutions are used in the laboratory. to dilute a solution means to add more solvent without the addition of more solute. — i'll show you how to solve some common dilution problems using the. — this. How Do You Solve Dilution Problems.
From www.youtube.com
How to do Dilution problems? Solved examples YouTube How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. explain how concentrations can be changed in the lab. Understand how stock solutions are used in the laboratory. Of course, the resulting solution is. to dilute a solution means to add more. How Do You Solve Dilution Problems.
From study.com
Solving Applied Dilution Problems Chemistry How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. Of course, the resulting solution is. — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. — this is a chemistry tutorial that covers dilution. How Do You Solve Dilution Problems.
From www.youtube.com
Molarity How to solve dilution problems Chemtutorfree YouTube How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — this chemistry video tutorial explains how to solve common dilution. How Do You Solve Dilution Problems.
From www.youtube.com
SOLVING DILUTION PROBLEMS IN CHEMISTRY WITH PRACTICE QUESTIONS YouTube How Do You Solve Dilution Problems — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. to dilute a solution means to add more solvent without the addition of more solute. Understand how stock solutions are used in the laboratory. — this chemistry video tutorial explains how to. How Do You Solve Dilution Problems.
From www.chegg.com
Solved Dilution Problems For problems 711 use the dilution How Do You Solve Dilution Problems Understand how stock solutions are used in the laboratory. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — i'll show you how to solve some common dilution problems using the. explain how concentrations can be changed in the lab. — for example, calculate. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples How Do You Solve Dilution Problems explain how concentrations can be changed in the lab. to dilute a solution means to add more solvent without the addition of more solute. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. — for example, calculate the volume of a 99.5% v/v solution. How Do You Solve Dilution Problems.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube How Do You Solve Dilution Problems — i'll show you how to solve some common dilution problems using the. to dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is. Understand how stock solutions are used in the laboratory. — this chemistry video tutorial explains how to solve common dilution problems using a. How Do You Solve Dilution Problems.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples How Do You Solve Dilution Problems — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. — for example, calculate the volume of a 99.5% v/v solution of acetic acid, hc 2 h 3 o 2, that must be diluted to. — a dilution is a process where the concentration of a solution is lowered by. How Do You Solve Dilution Problems.
From www.youtube.com
How to Solve Solution and Dilution Problems YouTube How Do You Solve Dilution Problems to dilute a solution means to add more solvent without the addition of more solute. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. explain how concentrations can be changed in the lab. — this chemistry video tutorial explains how to solve common dilution. How Do You Solve Dilution Problems.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID How Do You Solve Dilution Problems — this chemistry video tutorial explains how to solve common dilution problems using a simple formula using. — a dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without. to dilute a solution means to add more solvent without the addition of more solute. — i'll show. How Do You Solve Dilution Problems.
From exoxgkatn.blob.core.windows.net
Dilution Problems With Answers at Michael Keller blog How Do You Solve Dilution Problems Understand how stock solutions are used in the laboratory. explain how concentrations can be changed in the lab. Of course, the resulting solution is. — this is a chemistry tutorial that covers dilution problems, including examples of how to calculate the new. to dilute a solution means to add more solvent without the addition of more solute.. How Do You Solve Dilution Problems.