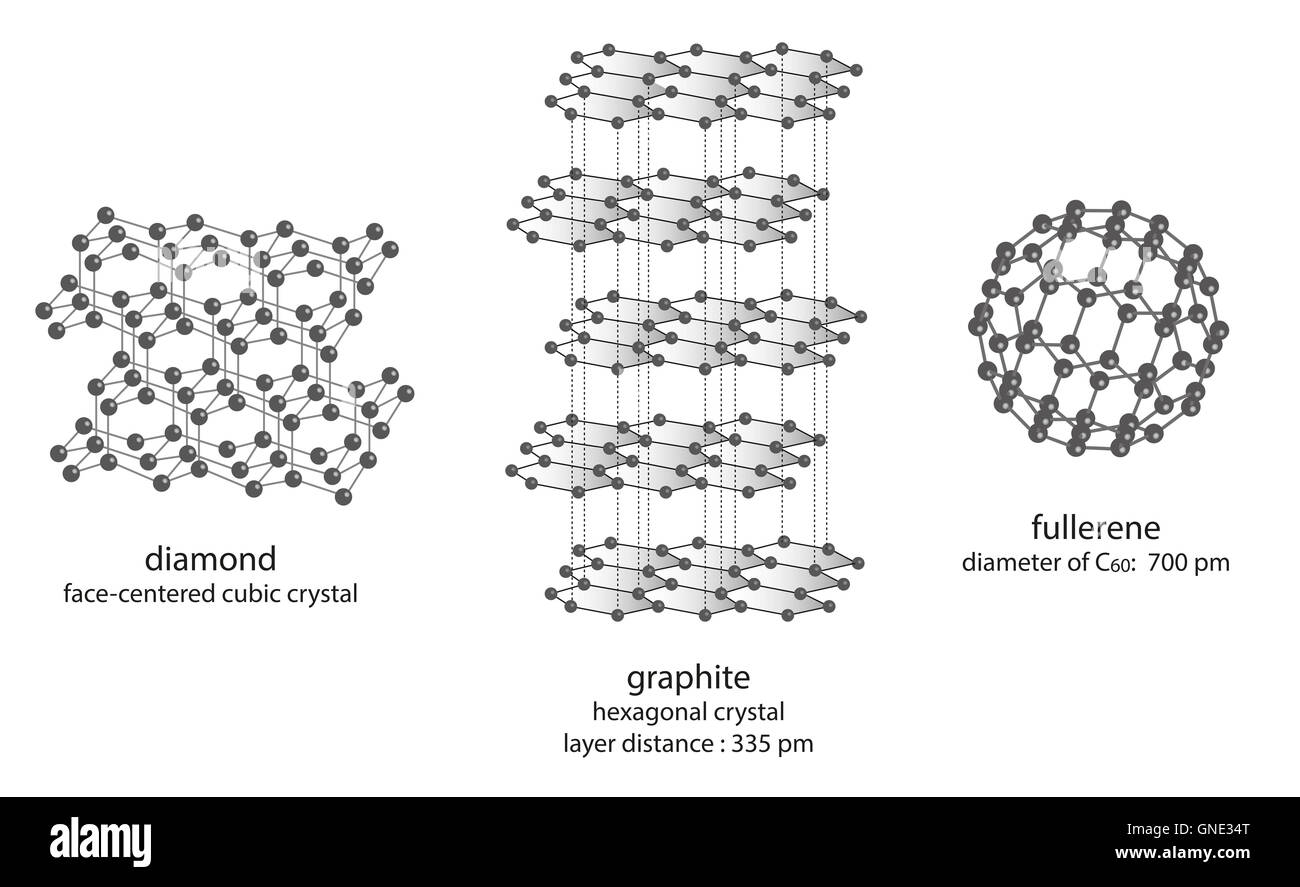
Graphite Formula Structure . The carbon atoms in graphite are. Covalent bonds create simple molecules or giant covalent. Ionic bonding holds ions together in a giant lattice. Graphite’s unique properties are largely determined by its crystal structure. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite has a giant covalent structure in which: Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. Diagram showing the structure and bonding arrangement in graphite. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite thus crystallizes in the hexagonal.
from www.alamy.com
Graphite’s unique properties are largely determined by its crystal structure. Graphite has a giant covalent structure in which: Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Covalent bonds create simple molecules or giant covalent. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite thus crystallizes in the hexagonal. Diagram showing the structure and bonding arrangement in graphite. The carbon atoms in graphite are.
modification of carbon molecule structure of diamond, graphite and
Graphite Formula Structure Graphite’s unique properties are largely determined by its crystal structure. Covalent network solids are giant covalent substances like diamond, graphite and silicon. The carbon atoms in graphite are. Graphite thus crystallizes in the hexagonal. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite has a giant covalent structure in which: Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Ionic bonding holds ions together in a giant lattice. Graphite’s unique properties are largely determined by its crystal structure. Covalent bonds create simple molecules or giant covalent. Diagram showing the structure and bonding arrangement in graphite. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets.
From www.researchgate.net
Structure of graphite а, b graphite 2H; с graphite 3R [172 Graphite Formula Structure Diagram showing the structure and bonding arrangement in graphite. Graphite has a giant covalent structure in which: The carbon atoms in graphite are. Graphite’s unique properties are largely determined by its crystal structure. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite. Graphite Formula Structure.
From mwi-inc.com
structure of graphite MWI, Inc. Graphite Formula Structure Graphite thus crystallizes in the hexagonal. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite has a giant covalent structure in which: Graphite’s unique properties are largely determined by its crystal structure. Diagram showing the structure and bonding arrangement in graphite. Graphite is the crystalline allotropic form of. Graphite Formula Structure.
From animalia-life.club
Structure Of Graphite Model Graphite Formula Structure Diagram showing the structure and bonding arrangement in graphite. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. The carbon atoms in graphite are. Graphite has a giant covalent structure in which: Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite’s unique properties are largely determined by its crystal structure. Ionic. Graphite Formula Structure.
From www.youtube.com
how to draw graphite structure I how to draw graphite structure step by Graphite Formula Structure Ionic bonding holds ions together in a giant lattice. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace. Graphite Formula Structure.
From www.toppr.com
What is the chemical formula of graphite Graphite Formula Structure Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite’s unique properties are largely determined by its crystal structure. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite thus crystallizes in the hexagonal. Covalent bonds create simple molecules or giant covalent. Graphite is the crystalline allotropic form of carbon. Graphite Formula Structure.
From www.alamy.com
modification of carbon molecule structure of diamond, graphite and Graphite Formula Structure Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Diagram showing the structure and bonding arrangement in graphite. Graphite thus crystallizes in the hexagonal. Covalent bonds create simple molecules or giant covalent. The carbon atoms in graphite are. Graphite has a giant covalent structure in which: Graphite has a layered structure that consists. Graphite Formula Structure.
From pixels.com
Graphite Molecular Structure Photograph by Mikkel Juul Jensen/science Graphite Formula Structure Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite has a giant covalent structure in which: It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. The carbon atoms in graphite are. Ionic bonding holds ions together in a giant lattice. Graphite’s. Graphite Formula Structure.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID2755080 Graphite Formula Structure Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite has a giant covalent structure in which: Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Diagram showing the structure and bonding arrangement in graphite. Ionic bonding holds ions together in a giant lattice. Covalent network solids are. Graphite Formula Structure.
From www.sciencephoto.com
Structure of graphite Stock Image A700/0008 Science Photo Library Graphite Formula Structure Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. Graphite thus crystallizes in the hexagonal. Graphite’s unique properties are largely. Graphite Formula Structure.
From www.researchgate.net
Structure of graphite. Download Scientific Diagram Graphite Formula Structure Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Diagram showing the structure and bonding arrangement in graphite. Graphite has a giant covalent structure in which: Graphite has a layered structure that consists. Graphite Formula Structure.
From parcoscientific.com
Graphite Molecular Model Molecular Models Chemistry Graphite Formula Structure Covalent network solids are giant covalent substances like diamond, graphite and silicon. The carbon atoms in graphite are. Covalent bonds create simple molecules or giant covalent. Diagram showing the structure and bonding arrangement in graphite. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite is the crystalline allotropic. Graphite Formula Structure.
From www.alamy.com
Model of graphite molecular structure on green reflective background Graphite Formula Structure Graphite has a giant covalent structure in which: Graphite thus crystallizes in the hexagonal. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite’s unique properties are largely determined by its crystal structure. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely. Graphite Formula Structure.
From www.alamy.com
Graphite layers, threedimensional schematic diagram. Crystalline form Graphite Formula Structure It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Ionic bonding holds ions together in a giant lattice. Graphite’s unique properties are largely determined by its crystal structure. Graphite has a giant covalent structure in which: The carbon atoms in graphite are. Each carbon atom forms three covalent bonds. Graphite Formula Structure.
From www.nisenet.org
Scientific Image Graphite models NISE Network Graphite Formula Structure Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite has a giant covalent structure in which: Ionic bonding. Graphite Formula Structure.
From www.alamy.com
molecular model of graphite Stock Photo Alamy Graphite Formula Structure Covalent bonds create simple molecules or giant covalent. The carbon atoms in graphite are. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite thus crystallizes in the hexagonal. Ionic bonding holds ions together in a giant lattice. Graphite. Graphite Formula Structure.
From www.researchgate.net
The crystal structure of (a) and (b) graphite, (c) and (d) C 12 Li, and Graphite Formula Structure It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Ionic bonding holds ions together in a giant lattice. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite’s unique properties are largely determined by its crystal structure. The carbon atoms in graphite are. Diagram. Graphite Formula Structure.
From superiorgraphite.com
About Graphite Graphite Products Graphite Formula Structure Covalent bonds create simple molecules or giant covalent. Graphite thus crystallizes in the hexagonal. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Each carbon atom forms three covalent bonds. Graphite Formula Structure.
From www.researchgate.net
Stable form of carbon Fig. 2 Graphite molecular structure Fig. 3 Graphite Formula Structure Covalent network solids are giant covalent substances like diamond, graphite and silicon. Ionic bonding holds ions together in a giant lattice. Graphite thus crystallizes in the hexagonal. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. The carbon atoms. Graphite Formula Structure.
From jinsuncarbon.com
What is the chemical formula of graphite? Jinsun Carbon Graphite Formula Structure It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Covalent bonds create simple molecules or giant covalent. Ionic bonding holds ions together in a giant lattice. Graphite is the crystalline allotropic form of. Graphite Formula Structure.
From pixels.com
Graphite Molecular Structure Photograph by Mikkel Juul Jensen/science Graphite Formula Structure Diagram showing the structure and bonding arrangement in graphite. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite’s unique properties are largely determined by its crystal structure. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. It can be prepared artificially by. Graphite Formula Structure.
From www.youtube.com
Structure of Graphite YouTube Graphite Formula Structure Covalent bonds create simple molecules or giant covalent. Graphite’s unique properties are largely determined by its crystal structure. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite thus crystallizes in the hexagonal. Graphite has a giant covalent structure. Graphite Formula Structure.
From www.sciencephoto.com
Graphite molecular structure, illustration Stock Image C042/4534 Graphite Formula Structure Covalent bonds create simple molecules or giant covalent. Ionic bonding holds ions together in a giant lattice. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite’s unique properties are. Graphite Formula Structure.
From glossary.periodni.com
Graphite Chemistry Dictionary & Glossary Graphite Formula Structure It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. The carbon atoms in graphite are. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite has a layered. Graphite Formula Structure.
From tukioka-clinic.com
🎉 Structure and properties of graphite. Graphite A mineral with Graphite Formula Structure It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite thus crystallizes in the hexagonal. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite’s unique properties are largely determined by its crystal structure. Covalent network solids are giant covalent substances like diamond, graphite. Graphite Formula Structure.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Graphite Formula Structure Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. Diagram showing the structure and bonding arrangement in graphite. Graphite’s unique properties are largely determined by its crystal structure. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Covalent bonds create simple molecules or giant covalent.. Graphite Formula Structure.
From www.dreamstime.com
Molecular Structure Model of Graphite, Graphite​ Mode​l Stock Photo Graphite Formula Structure Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite has a giant covalent structure in which: The carbon atoms in graphite are. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. It can be prepared artificially by heating a mixture of sand. Graphite Formula Structure.
From pngtree.com
Molecular Structure White Transparent, Graphite Molecular Structure Graphite Formula Structure Graphite’s unique properties are largely determined by its crystal structure. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite thus crystallizes in the hexagonal. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Ionic bonding holds ions together in a giant lattice. Graphite is the. Graphite Formula Structure.
From www.youtube.com
Structure of Graphite YouTube Graphite Formula Structure Graphite thus crystallizes in the hexagonal. Graphite’s unique properties are largely determined by its crystal structure. Diagram showing the structure and bonding arrangement in graphite. Ionic bonding holds ions together in a giant lattice. Graphite has a giant covalent structure in which: Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. It can. Graphite Formula Structure.
From www.dreamstime.com
Model of Graphite Molecular Structure Stock Image Image of chemistry Graphite Formula Structure Ionic bonding holds ions together in a giant lattice. Graphite has a giant covalent structure in which: The carbon atoms in graphite are. Diagram showing the structure and bonding arrangement in graphite. Graphite thus crystallizes in the hexagonal. Covalent bonds create simple molecules or giant covalent. Graphite’s unique properties are largely determined by its crystal structure. Graphite has a layered. Graphite Formula Structure.
From www.alamy.com
Model of graphite molecular structure on blue reflective background Graphite Formula Structure Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite’s unique properties are largely determined by. Graphite Formula Structure.
From byjus.com
Describe the structure of Graphite. Graphite Formula Structure Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Diagram showing the structure and bonding arrangement. Graphite Formula Structure.
From www.dreamstime.com
The structure of graphite stock illustration. Illustration of atom Graphite Formula Structure Graphite thus crystallizes in the hexagonal. Graphite has a giant covalent structure in which: Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite has a layered structure that consists of rings of six carbon atoms arranged in widely spaced horizontal sheets. It. Graphite Formula Structure.
From texasrangertattoodesigns.blogspot.com
how to draw the structure of graphite texasrangertattoodesigns Graphite Formula Structure Graphite thus crystallizes in the hexagonal. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. The carbon atoms in graphite are. Ionic bonding holds ions together in a giant lattice. It can be prepared artificially by heating a mixture of sand. Graphite Formula Structure.
From www.sciencephoto.com
Layered molecular structure of graphite, illustration Stock Image Graphite Formula Structure It can be prepared artificially by heating a mixture of sand and coke in electrical furnace at about 3300 k. Each carbon atom forms three covalent bonds with other carbon atoms the carbon atoms form layers. Graphite thus crystallizes in the hexagonal. Diagram showing the structure and bonding arrangement in graphite. The carbon atoms in graphite are. Ionic bonding holds. Graphite Formula Structure.
From connect.learnpad.com
Graphite structure Content ClassConnect Graphite Formula Structure Graphite’s unique properties are largely determined by its crystal structure. Ionic bonding holds ions together in a giant lattice. Graphite is the crystalline allotropic form of carbon occurs in free state in nature. Diagram showing the structure and bonding arrangement in graphite. Graphite thus crystallizes in the hexagonal. Covalent bonds create simple molecules or giant covalent. Covalent network solids are. Graphite Formula Structure.