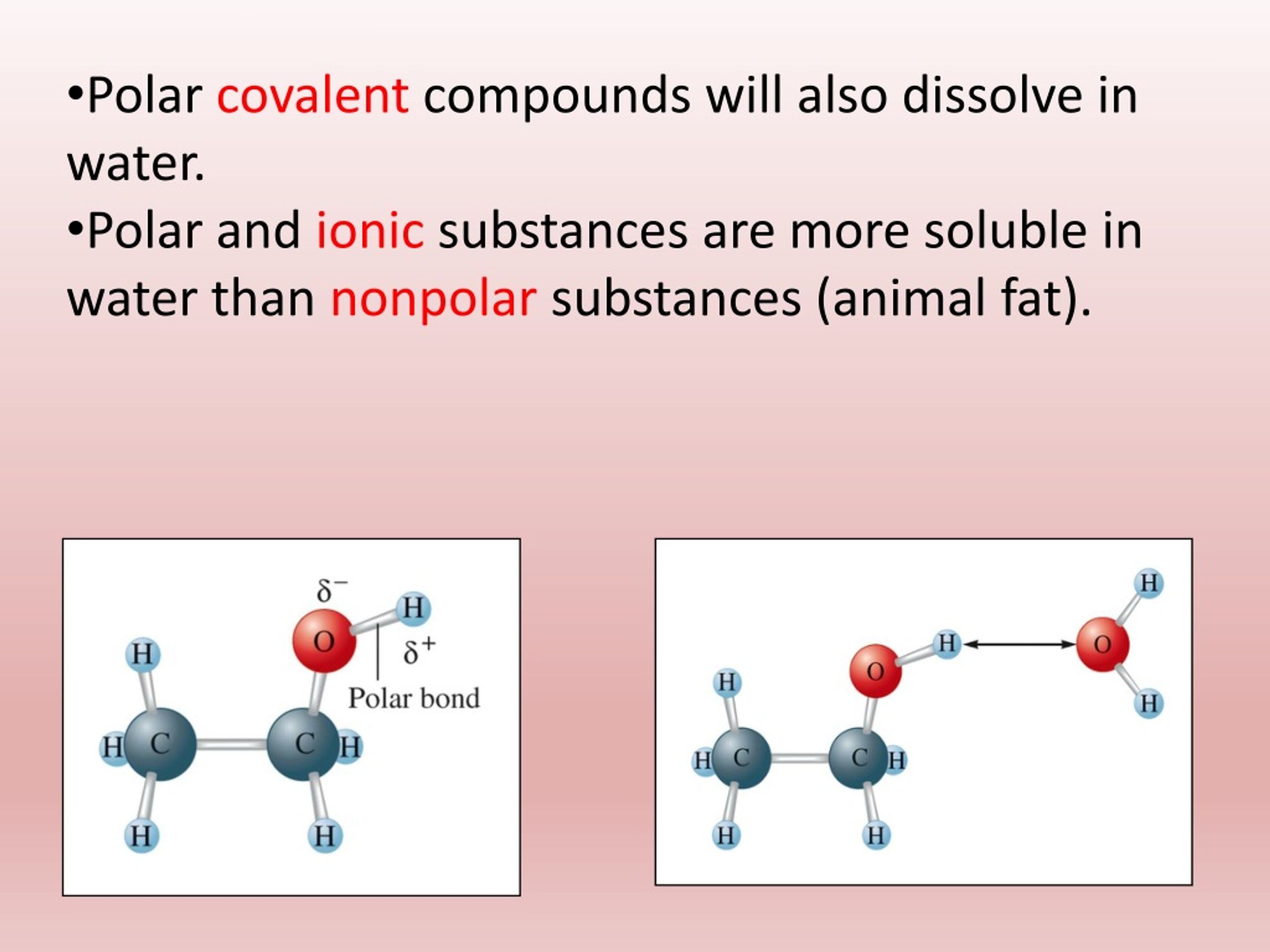
What Happens When You Mix Polar And Nonpolar Substances . When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Nonpolar substances are likely to. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Polar substances are likely to dissolve in polar solvents. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of.
from www.slideserve.com
For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Nonpolar substances are likely to. Polar substances are likely to dissolve in polar solvents. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed.
PPT Solutions and Mixtures PowerPoint Presentation, free download
What Happens When You Mix Polar And Nonpolar Substances Nonpolar substances are likely to. Nonpolar substances are likely to. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Polar substances are likely to dissolve in polar solvents. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the.
From www.pinterest.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii Teaching What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Polar substances are likely to dissolve in polar solvents. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Note that every time charged particles (ionic. What Happens When You Mix Polar And Nonpolar Substances.
From www.youtube.com
What is the Difference Between Polar and Non Polar Substances What Happens When You Mix Polar And Nonpolar Substances Polar substances are likely to dissolve in polar solvents. Nonpolar substances are likely to. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Note that every time charged particles (ionic compounds or polar substances) are. What Happens When You Mix Polar And Nonpolar Substances.
From www.pinterest.com
HF is Polar or Nonpolar Covalent Bond Covalent bonding, What happens What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed.. What Happens When You Mix Polar And Nonpolar Substances.
From simp-link.com
Difference between polar and nonpolar examples What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Nonpolar substances are likely to. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. For example, ionic compounds, which are very polar, are often soluble in. What Happens When You Mix Polar And Nonpolar Substances.
From www.numerade.com
SOLVED{c) BFz (d) SOz ionic ionic nonpolar covalent nonpolar covalent What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Nonpolar substances are likely to. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Polar substances are likely to dissolve in polar solvents. Note that every time charged. What Happens When You Mix Polar And Nonpolar Substances.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary What Happens When You Mix Polar And Nonpolar Substances According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Note that every time charged particles (ionic. What Happens When You Mix Polar And Nonpolar Substances.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Happens When You Mix Polar And Nonpolar Substances For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Nonpolar substances are likely to. When two nonpolar molecules. What Happens When You Mix Polar And Nonpolar Substances.
From studyloadstones.z21.web.core.windows.net
Why Does Nonpolar Dissolve Nonpolar What Happens When You Mix Polar And Nonpolar Substances For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Nonpolar substances are likely to. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. When two nonpolar molecules. What Happens When You Mix Polar And Nonpolar Substances.
From courses.lumenlearning.com
The Dissolution Process Chemistry What Happens When You Mix Polar And Nonpolar Substances For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. According to the theory of london dispersion forces, intermolecular. What Happens When You Mix Polar And Nonpolar Substances.
From www.expii.com
Polar vs. Nonpolar Bonds — Overview & Examples Expii What Happens When You Mix Polar And Nonpolar Substances For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Polar. What Happens When You Mix Polar And Nonpolar Substances.
From bayleegroconner.blogspot.com
Is Water Polar or Nonpolar BayleegroConner What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Polar substances are likely to dissolve in polar solvents. Note that every time charged particles (ionic. What Happens When You Mix Polar And Nonpolar Substances.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 What Happens When You Mix Polar And Nonpolar Substances For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Nonpolar substances are likely to. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Polar substances. What Happens When You Mix Polar And Nonpolar Substances.
From www.youtube.com
Polar vs Nonpolar molecules How to tell? [GCE A Level Chemistry] YouTube What Happens When You Mix Polar And Nonpolar Substances Nonpolar substances are likely to. Polar substances are likely to dissolve in polar solvents. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. According to the theory of. What Happens When You Mix Polar And Nonpolar Substances.
From www.youtube.com
What is the difference between polar and nonpolar covalent bonds? YouTube What Happens When You Mix Polar And Nonpolar Substances Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Polar substances are likely to dissolve in polar solvents. For example, ionic compounds, which are very polar, are often soluble in the polar. What Happens When You Mix Polar And Nonpolar Substances.
From www.britannica.com
covalent bond Definition, Properties, Examples, & Facts Britannica What Happens When You Mix Polar And Nonpolar Substances According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Polar substances are likely to dissolve in polar solvents. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Nonpolar substances are likely to. For example, ionic compounds, which are very polar, are often. What Happens When You Mix Polar And Nonpolar Substances.
From slideplayer.com
Classification of Matter and Solutions Notes Part 1 ppt download What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed.. What Happens When You Mix Polar And Nonpolar Substances.
From www.numerade.com
SOLVED Wivy is water always tested in each procedure? Compare and What Happens When You Mix Polar And Nonpolar Substances Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Polar substances. What Happens When You Mix Polar And Nonpolar Substances.
From slideplayer.com
Bellwork 1. What do you remember about the difference between What Happens When You Mix Polar And Nonpolar Substances For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. When two nonpolar molecules are mixed together,. What Happens When You Mix Polar And Nonpolar Substances.
From www.pw.live
Difference Between Polar And Nonpolar What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Nonpolar substances are likely to. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Polar substances are likely to dissolve in polar solvents. According to the. What Happens When You Mix Polar And Nonpolar Substances.
From www.coscinecreative.com
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative What Happens When You Mix Polar And Nonpolar Substances Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar substances are likely to dissolve in polar solvents. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction. What Happens When You Mix Polar And Nonpolar Substances.
From sciencenotes.org
Polar and Nonpolar Molecules What Happens When You Mix Polar And Nonpolar Substances Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Nonpolar substances are likely to. Polar substances are likely to dissolve in polar solvents. Note that every time charged particles (ionic compounds or polar substances) are. What Happens When You Mix Polar And Nonpolar Substances.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download What Happens When You Mix Polar And Nonpolar Substances According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Nonpolar substances are likely to. For example, ionic compounds, which are very polar, are often soluble. What Happens When You Mix Polar And Nonpolar Substances.
From stilleducation.com
Polar and Nonpolar Covalent Bonds Characteristics & Differences What Happens When You Mix Polar And Nonpolar Substances Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. When two nonpolar molecules are mixed together, neither requires. What Happens When You Mix Polar And Nonpolar Substances.
From pediaa.com
Difference Between Polar and Nonpolar Molecules Definition, Formation What Happens When You Mix Polar And Nonpolar Substances Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Polar substances are likely to dissolve in polar solvents. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction. What Happens When You Mix Polar And Nonpolar Substances.
From www.slideserve.com
PPT Chapter 2 Aqueous Chemistry PowerPoint Presentation, free What Happens When You Mix Polar And Nonpolar Substances Polar substances are likely to dissolve in polar solvents. Nonpolar substances are likely to. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. When two nonpolar molecules are mixed together, neither requires a charged region for. What Happens When You Mix Polar And Nonpolar Substances.
From www.slideserve.com
PPT ELECTRONEGATIVITY POLAR BONDS MOLECULAR POLARITY PowerPoint What Happens When You Mix Polar And Nonpolar Substances According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar substances are likely to dissolve in polar solvents. For example, ionic compounds, which are very polar, are often soluble in the polar solvent. What Happens When You Mix Polar And Nonpolar Substances.
From www.youtube.com
Polar and Nonpolar Molecules YouTube What Happens When You Mix Polar And Nonpolar Substances Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar substances are likely to dissolve in polar solvents. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water.. What Happens When You Mix Polar And Nonpolar Substances.
From slideplayer.com
UNIT 6 SOLUTIONS AND GASES 6.2 What factors affect solubility? ppt What Happens When You Mix Polar And Nonpolar Substances When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Polar substances are likely to dissolve in polar solvents. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. According to the theory of london dispersion forces, intermolecular forces. What Happens When You Mix Polar And Nonpolar Substances.
From www.slideserve.com
PPT Chapter 10 Structures of Solids and Liquids PowerPoint What Happens When You Mix Polar And Nonpolar Substances Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar substances are likely to dissolve in polar solvents. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances. What Happens When You Mix Polar And Nonpolar Substances.
From www.numerade.com
SOLVED 18. Iodine dissolves in water, but its solubility in a nonpolar What Happens When You Mix Polar And Nonpolar Substances Nonpolar substances are likely to. Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar. What Happens When You Mix Polar And Nonpolar Substances.
From brainly.ph
give five descriptions of a polar and nonpolar molecule Brainly.ph What Happens When You Mix Polar And Nonpolar Substances Polar substances do not mix with nonpolar substances because the interactions between polar molecules are stronger than the. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed.. What Happens When You Mix Polar And Nonpolar Substances.
From studylib.net
Polar and Nonpolar Molecules What Happens When You Mix Polar And Nonpolar Substances Note that every time charged particles (ionic compounds or polar substances) are mixed, a solution is formed. Polar substances are likely to dissolve in polar solvents. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and. What Happens When You Mix Polar And Nonpolar Substances.
From ask.modifiyegaraj.com
Is Pcl5 Polar Or Nonpolar Asking List What Happens When You Mix Polar And Nonpolar Substances For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a.. What Happens When You Mix Polar And Nonpolar Substances.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 What Happens When You Mix Polar And Nonpolar Substances Polar substances are likely to dissolve in polar solvents. According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Note that every time charged particles (ionic. What Happens When You Mix Polar And Nonpolar Substances.
From www.coscinecreative.com
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative What Happens When You Mix Polar And Nonpolar Substances According to the theory of london dispersion forces, intermolecular forces are created in non polar substances by induction of. Nonpolar substances are likely to. When two nonpolar molecules are mixed together, neither requires a charged region for attraction and they are thus able to mix freely and form a. Note that every time charged particles (ionic compounds or polar substances). What Happens When You Mix Polar And Nonpolar Substances.