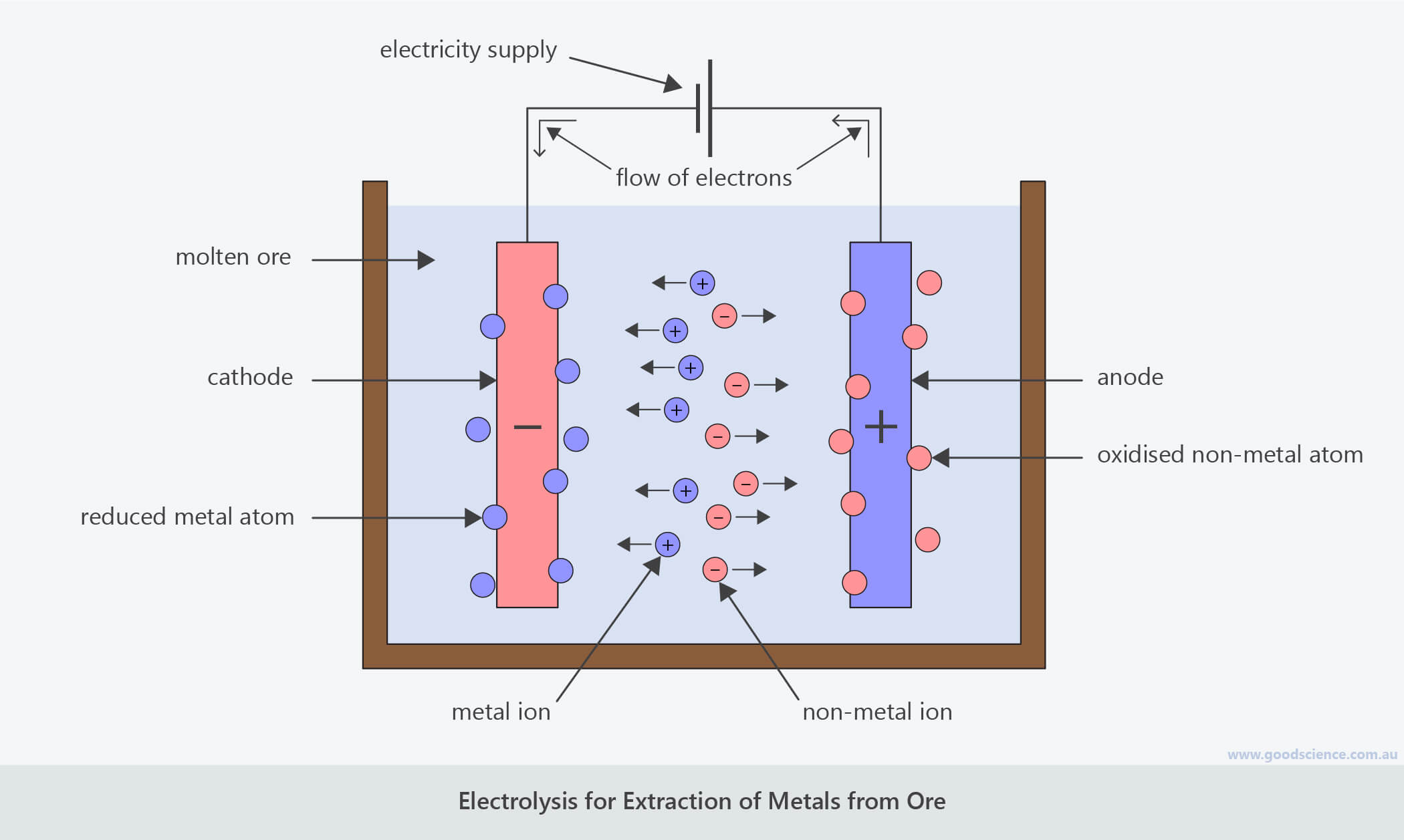
Electrode Used In Electrolysis Process . Reactive metals are extracted from their ores using electrolysis. Cathode is the negative electrode of an electrolysis cell. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. Na + ions combine with the free. Anion is a negatively charged ion which is attracted to the anode. Anode is the positive electrode of an electrolysis cell. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Ionic compounds conduct electricity when molten or in solution. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and.
from mungfali.com
Anode is the positive electrode of an electrolysis cell. Ionic compounds conduct electricity when molten or in solution. Cathode is the negative electrode of an electrolysis cell. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Na + ions combine with the free. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Anion is a negatively charged ion which is attracted to the anode. Reactive metals are extracted from their ores using electrolysis. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and.
Electrolysis Process Diagram
Electrode Used In Electrolysis Process Cathode is the negative electrode of an electrolysis cell. Anion is a negatively charged ion which is attracted to the anode. Cathode is the negative electrode of an electrolysis cell. Ionic compounds conduct electricity when molten or in solution. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Na + ions combine with the free. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. Anode is the positive electrode of an electrolysis cell. Reactive metals are extracted from their ores using electrolysis.
From byjus.com
With the help of a diagram explain the method of refining of copper by Electrode Used In Electrolysis Process Ionic compounds conduct electricity when molten or in solution. Anode is the positive electrode of an electrolysis cell. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held. Electrode Used In Electrolysis Process.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Electrode Used In Electrolysis Process These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Na + ions combine with the free. Reactive metals are extracted from their ores using electrolysis. Anion is a negatively charged ion which is attracted to the anode. Anode is the positive electrode of an electrolysis cell. The process. Electrode Used In Electrolysis Process.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? Electrode Used In Electrolysis Process The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Reactive metals are extracted from their ores using electrolysis. Anion is a negatively charged ion which is attracted to the anode. Na + ions combine with the free. The process is carried out in an electrolytic cell, an apparatus consisting. Electrode Used In Electrolysis Process.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Electrode Used In Electrolysis Process Reactive metals are extracted from their ores using electrolysis. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. Ionic compounds conduct electricity when molten or. Electrode Used In Electrolysis Process.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrode Used In Electrolysis Process These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. Anion is a negatively charged ion which is attracted to the anode. Cathode is the. Electrode Used In Electrolysis Process.
From owlcation.com
Electrolysis The Way of the Future Owlcation Electrode Used In Electrolysis Process Na + ions combine with the free. Anion is a negatively charged ion which is attracted to the anode. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Cathode is the negative electrode of an electrolysis cell. In a process called electroplating, a layer of a second metal. Electrode Used In Electrolysis Process.
From www.researchgate.net
Schematic of the membrane electrode assembly (MEA) and catalyst Electrode Used In Electrolysis Process The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. Anode is the positive electrode of an electrolysis cell. Anion is a negatively charged ion which. Electrode Used In Electrolysis Process.
From webmis.highland.cc.il.us
Electrolysis Electrode Used In Electrolysis Process Na + ions combine with the free. Anode is the positive electrode of an electrolysis cell. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. Reactive metals are extracted from their ores using electrolysis. The choice of electrodes used in electrolysis plays a crucial role. Electrode Used In Electrolysis Process.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrode Used In Electrolysis Process Ionic compounds conduct electricity when molten or in solution. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Na + ions combine with the free. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Anion is a. Electrode Used In Electrolysis Process.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Electrode Used In Electrolysis Process Cathode is the negative electrode of an electrolysis cell. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Anion is a negatively charged ion which is attracted to the anode. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in. Electrode Used In Electrolysis Process.
From www.researchgate.net
Schematic diagram of the alkaline electrolysis cell [34]. Download Electrode Used In Electrolysis Process Na + ions combine with the free. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Anode is the positive electrode of an electrolysis cell. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Cathode is the. Electrode Used In Electrolysis Process.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki Electrode Used In Electrolysis Process In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Anode is the positive electrode of an electrolysis cell. Anion is a negatively charged ion which. Electrode Used In Electrolysis Process.
From study.com
Electrolysis of Aqueous Solutions Lesson Electrode Used In Electrolysis Process These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Anion is a negatively charged ion which is attracted to the anode. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Ionic compounds conduct electricity when molten or. Electrode Used In Electrolysis Process.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace Electrode Used In Electrolysis Process The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Anion is a negatively charged ion which is attracted to the anode. Ionic compounds conduct electricity when molten or in solution. Na + ions combine with the free. In a process called electroplating, a layer of a second metal is. Electrode Used In Electrolysis Process.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process Electrode Used In Electrolysis Process These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Cathode is the negative electrode of an electrolysis cell. Anion is a negatively charged ion which is attracted to the anode. Anode is the positive electrode of an electrolysis cell. Reactive metals are extracted from their ores using electrolysis.. Electrode Used In Electrolysis Process.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis Electrode Used In Electrolysis Process Ionic compounds conduct electricity when molten or in solution. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Reactive metals are extracted from their ores using electrolysis. In. Electrode Used In Electrolysis Process.
From mungfali.com
Electrolysis Process Diagram Electrode Used In Electrolysis Process Cathode is the negative electrode of an electrolysis cell. Reactive metals are extracted from their ores using electrolysis. Na + ions combine with the free. Ionic compounds conduct electricity when molten or in solution. Anode is the positive electrode of an electrolysis cell. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na +. Electrode Used In Electrolysis Process.
From mavink.com
Labelled Diagram Of Electrolysis Electrode Used In Electrolysis Process Anion is a negatively charged ion which is attracted to the anode. Cathode is the negative electrode of an electrolysis cell. Na + ions combine with the free. Ionic compounds conduct electricity when molten or in solution. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during. Electrode Used In Electrolysis Process.
From uen.pressbooks.pub
Electrolysis Introductory Chemistry Electrode Used In Electrolysis Process Anode is the positive electrode of an electrolysis cell. Ionic compounds conduct electricity when molten or in solution. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held. Electrode Used In Electrolysis Process.
From energy.gov
Hydrogen Production Electrolysis Department of Energy Electrode Used In Electrolysis Process The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts. Electrode Used In Electrolysis Process.
From mungfali.com
Electrolysis Process Diagram Electrode Used In Electrolysis Process Anion is a negatively charged ion which is attracted to the anode. Anode is the positive electrode of an electrolysis cell. Cathode is the negative electrode of an electrolysis cell. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. These negative electrons create a negative electrode in the electrolytic. Electrode Used In Electrolysis Process.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrode Used In Electrolysis Process The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Anode is the positive electrode of an electrolysis cell. Na + ions combine with the free. Ionic compounds conduct electricity when molten or in solution. In a process called electroplating, a layer of a second metal is deposited on the. Electrode Used In Electrolysis Process.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrode Used In Electrolysis Process Reactive metals are extracted from their ores using electrolysis. Anion is a negatively charged ion which is attracted to the anode. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of. Electrode Used In Electrolysis Process.
From byjus.com
Water Electrolysis Principle of Water Electrolysis, Important Factors Electrode Used In Electrolysis Process Anode is the positive electrode of an electrolysis cell. Na + ions combine with the free. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Anion is a. Electrode Used In Electrolysis Process.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download Electrode Used In Electrolysis Process Anion is a negatively charged ion which is attracted to the anode. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Reactive metals are extracted from their ores using electrolysis. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that. Electrode Used In Electrolysis Process.
From www.snexplores.org
Explainer What is an electrode? Electrode Used In Electrolysis Process Cathode is the negative electrode of an electrolysis cell. Ionic compounds conduct electricity when molten or in solution. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Anion is a negatively charged ion which is attracted to the anode. The process is carried out in an electrolytic cell, an. Electrode Used In Electrolysis Process.
From www.ifam.fraunhofer.de
Electrolysis Electrode Used In Electrolysis Process The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Anode is the positive electrode of an electrolysis cell. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Ionic compounds conduct electricity when molten or in solution. Cathode is. Electrode Used In Electrolysis Process.
From www.researchgate.net
Configurations for water electrolysis (a) proton exchange membrane Electrode Used In Electrolysis Process Anion is a negatively charged ion which is attracted to the anode. In a process called electroplating, a layer of a second metal is deposited on the metal electrode that acts as the cathode during electrolysis. Cathode is the negative electrode of an electrolysis cell. Reactive metals are extracted from their ores using electrolysis. The process is carried out in. Electrode Used In Electrolysis Process.
From www.researchgate.net
Schematic diagram of electrolysis process. Download Scientific Diagram Electrode Used In Electrolysis Process Anode is the positive electrode of an electrolysis cell. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Cathode is the negative electrode of an electrolysis cell. Na + ions combine with the free. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes. Electrode Used In Electrolysis Process.
From www.oceangeothermal.org
Hydrogen Through Electrolysis Ocean Geothermal Energy Foundation Electrode Used In Electrolysis Process These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Cathode is the negative electrode of an electrolysis cell. Anion is a negatively charged ion which is attracted to the anode. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of. Electrode Used In Electrolysis Process.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrode Used In Electrolysis Process Anion is a negatively charged ion which is attracted to the anode. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. Na + ions combine with the free. Anode is the positive electrode of an electrolysis cell. Cathode is the negative electrode of an electrolysis cell. In a. Electrode Used In Electrolysis Process.
From www.chemistrylearner.com
Electrolysis of Water Definition and Equation Electrode Used In Electrolysis Process These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Cathode is the negative electrode of an electrolysis cell. Na + ions combine with the free. Ionic compounds conduct. Electrode Used In Electrolysis Process.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Electrode Used In Electrolysis Process Anode is the positive electrode of an electrolysis cell. Reactive metals are extracted from their ores using electrolysis. The process is carried out in an electrolytic cell, an apparatus consisting of positive and negative electrodes held apart and. Na + ions combine with the free. Ionic compounds conduct electricity when molten or in solution. In a process called electroplating, a. Electrode Used In Electrolysis Process.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Electrode Used In Electrolysis Process Ionic compounds conduct electricity when molten or in solution. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. Anode is the positive electrode of an electrolysis cell. Reactive metals are extracted from their ores using electrolysis. Anion is a negatively charged ion which is attracted to the anode. In. Electrode Used In Electrolysis Process.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry Electrode Used In Electrolysis Process Na + ions combine with the free. These negative electrons create a negative electrode in the electrolytic cell, which attracts the positive na + ions in the electrolyte. The choice of electrodes used in electrolysis plays a crucial role in determining the efficiency and effectiveness of the process. The process is carried out in an electrolytic cell, an apparatus consisting. Electrode Used In Electrolysis Process.