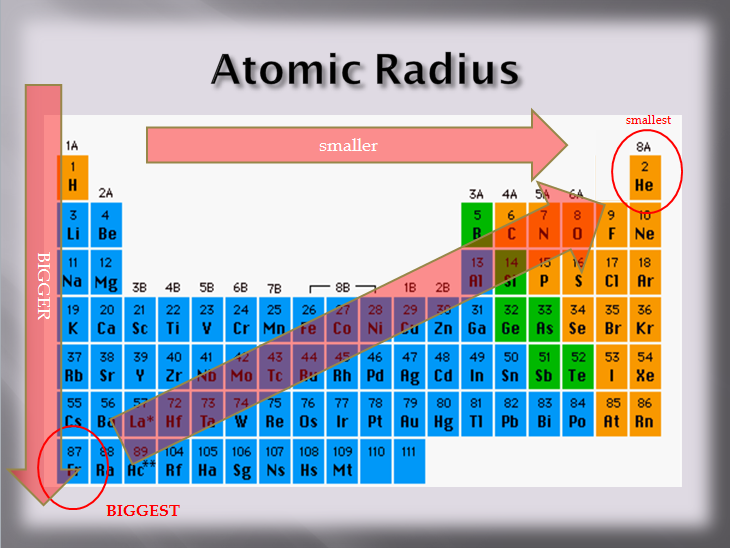
Smallest Atomic Size . This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. 119 rows access detailed info on all elements: Each atom’s size is relative to the largest element, cesium. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. It follows that the smallest atoms derive the right of the table as we face it. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. Atom size values are calculated from atomic radius data. Atomic mass, electron configurations, charges, and more. Excluding the noble gases, the smaller atoms from the right hand side, i.e. The atomic radius is an indication of the size of an atom. As a result, atoms and ions cannot be said to have exact sizes. In this section, we discuss how atomic and ion “sizes” are defined and obtained.
from utedzz.blogspot.com
The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. The atomic radius is an indication of the size of an atom. In this section, we discuss how atomic and ion “sizes” are defined and obtained. Excluding the noble gases, the smaller atoms from the right hand side, i.e. As a result, atoms and ions cannot be said to have exact sizes. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more.
Periodic Table Largest To Smallest Atomic Radius Periodic Table Timeline
Smallest Atomic Size It follows that the smallest atoms derive the right of the table as we face it. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. Atomic mass, electron configurations, charges, and more. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. It follows that the smallest atoms derive the right of the table as we face it. As a result, atoms and ions cannot be said to have exact sizes. The atomic radius is an indication of the size of an atom. In this section, we discuss how atomic and ion “sizes” are defined and obtained. 119 rows access detailed info on all elements: Atom size values are calculated from atomic radius data. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Each atom’s size is relative to the largest element, cesium. Excluding the noble gases, the smaller atoms from the right hand side, i.e.
From jordrail.weebly.com
Smallest atomic radius jordrail Smallest Atomic Size It follows that the smallest atoms derive the right of the table as we face it. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. 119 rows the atomic radius of a chemical element is the distance from the center of the. Smallest Atomic Size.
From www.vrogue.co
Periodic Table Of The Elements Atomic Radius vrogue.co Smallest Atomic Size Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. 119 rows access detailed info on all elements: As a result, atoms and ions cannot be said to have exact sizes. Excluding the noble gases, the smaller atoms from the right hand side, i.e. This table shows. Smallest Atomic Size.
From sciencenotes.org
Downloadable Periodic Table Circle Tiles Smallest Atomic Size Excluding the noble gases, the smaller atoms from the right hand side, i.e. 119 rows access detailed info on all elements: Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. The atomic radius of the elements increases as we go from right to left across a. Smallest Atomic Size.
From www.doubtnut.com
Write down the electronic configuration of the following elements from Smallest Atomic Size As a result, atoms and ions cannot be said to have exact sizes. Atomic mass, electron configurations, charges, and more. 119 rows access detailed info on all elements: The atomic radius is an indication of the size of an atom. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have. Smallest Atomic Size.
From owlcation.com
The Wonders of the Periodic Table Owlcation Smallest Atomic Size 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. It follows that the smallest atoms derive the right of the table as we face it. The atomic radius is. Smallest Atomic Size.
From www.researchgate.net
Periodic table of atomic sizes used in molecular models. ( A ) CPK Smallest Atomic Size It follows that the smallest atoms derive the right of the table as we face it. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell. Smallest Atomic Size.
From freyabartlett.z13.web.core.windows.net
Size Of Atoms Chart Smallest Atomic Size In this section, we discuss how atomic and ion “sizes” are defined and obtained. Atomic mass, electron configurations, charges, and more. As a result, atoms and ions cannot be said to have exact sizes. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. Atom size values. Smallest Atomic Size.
From chem.libretexts.org
3.1 Ionic Atoms Chemistry LibreTexts Smallest Atomic Size 119 rows access detailed info on all elements: The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. Atom size. Smallest Atomic Size.
From ar.inspiredpencil.com
Atomic Size Chart Smallest Atomic Size It follows that the smallest atoms derive the right of the table as we face it. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. This table shows how the atom size, and atomic radius values change as you move horizontally and. Smallest Atomic Size.
From www.periodictableprintable.com
Periodic Table Decreasing Atomic Radius 2024 Periodic Table Printable Smallest Atomic Size Excluding the noble gases, the smaller atoms from the right hand side, i.e. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. As a result, atoms and ions cannot be said to have exact sizes. Each atom’s size is relative to the largest element,. Smallest Atomic Size.
From anatomyandphysiologyi.com
Atoms and Elements Anatomy & Physiology Smallest Atomic Size The atomic radius is an indication of the size of an atom. It follows that the smallest atoms derive the right of the table as we face it. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. In this section, we discuss how atomic and ion. Smallest Atomic Size.
From utedzz.blogspot.com
Periodic Table Largest To Smallest Atomic Radius Periodic Table Timeline Smallest Atomic Size Excluding the noble gases, the smaller atoms from the right hand side, i.e. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the. Smallest Atomic Size.
From www.chegg.com
Solved Select the element with the smallest atomic size Smallest Atomic Size It follows that the smallest atoms derive the right of the table as we face it. The atomic radius is an indication of the size of an atom. In this section, we discuss how atomic and ion “sizes” are defined and obtained. The atomic radius of the elements increases as we go from right to left across a period and. Smallest Atomic Size.
From www.slideserve.com
PPT Anatomy & Physiology Basic Chemistry Chapter 2 PowerPoint Smallest Atomic Size This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. In this section, we discuss how atomic and ion “sizes” are defined and obtained. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. 119. Smallest Atomic Size.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation Smallest Atomic Size Each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. The atomic radius of the elements increases as we go from right to left across a period and as. Smallest Atomic Size.
From www.chem.fsu.edu
Electron Configurations Smallest Atomic Size It follows that the smallest atoms derive the right of the table as we face it. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. Atom size values are calculated from atomic radius data. In this section, we discuss how atomic and. Smallest Atomic Size.
From stock.adobe.com
Table showing electron orbital configuration of the smallest atoms Smallest Atomic Size The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Although the concept of a definite radius of. Smallest Atomic Size.
From payscalechart.z28.web.core.windows.net
atomic scale chart Table periodic chemistry elements chemicool Smallest Atomic Size The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. It follows that the smallest atoms derive the right of the table as we face it. Each atom’s size is relative to the largest element, cesium. 119 rows access detailed info on all. Smallest Atomic Size.
From socratic.org
For a given Period, which is the smallest atom? Socratic Smallest Atomic Size Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. As a result, atoms and ions cannot be said to have exact sizes. In this. Smallest Atomic Size.
From www.numerade.com
⏩SOLVEDIn each of the following sets of elements, indicate which Smallest Atomic Size The atomic radius is an indication of the size of an atom. As a result, atoms and ions cannot be said to have exact sizes. Each atom’s size is relative to the largest element, cesium. Atom size values are calculated from atomic radius data. In this section, we discuss how atomic and ion “sizes” are defined and obtained. Although the. Smallest Atomic Size.
From www.shutterstock.com
Atomic Sizes Periodic System Elements Stock Illustration 339037583 Smallest Atomic Size Excluding the noble gases, the smaller atoms from the right hand side, i.e. Atom size values are calculated from atomic radius data. As a result, atoms and ions cannot be said to have exact sizes. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. It follows that. Smallest Atomic Size.
From www.doubtnut.com
Which elements have the largest and the smallest atoms? Smallest Atomic Size Atomic mass, electron configurations, charges, and more. It follows that the smallest atoms derive the right of the table as we face it. As a result, atoms and ions cannot be said to have exact sizes. In this section, we discuss how atomic and ion “sizes” are defined and obtained. 119 rows access detailed info on all elements: Atom size. Smallest Atomic Size.
From www.alamy.com
3D Illustration Atomic structure. Atom is the smallest level of matter Smallest Atomic Size This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. Each atom’s size is relative to the largest element, cesium. As a result, atoms and ions cannot be said to have exact sizes. The atomic radius is an indication of the size of an atom. In this section,. Smallest Atomic Size.
From courses.lumenlearning.com
Atomic Size Introduction to Chemistry Smallest Atomic Size As a result, atoms and ions cannot be said to have exact sizes. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. In this section, we discuss how atomic and ion “sizes” are defined and obtained. This table shows how the atom size, and atomic radius. Smallest Atomic Size.
From webmis.highland.cc.il.us
Sizes of Atoms and Ions Smallest Atomic Size Atomic mass, electron configurations, charges, and more. In this section, we discuss how atomic and ion “sizes” are defined and obtained. The atomic radius is an indication of the size of an atom. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Although the. Smallest Atomic Size.
From www.learnatnoon.com
Atomic size and atomic radius explained Noon Academy Smallest Atomic Size Excluding the noble gases, the smaller atoms from the right hand side, i.e. 119 rows access detailed info on all elements: Each atom’s size is relative to the largest element, cesium. The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. This table. Smallest Atomic Size.
From chemistry.stackexchange.com
electrons Which atom is the smallest atom? Chemistry Stack Exchange Smallest Atomic Size 119 rows access detailed info on all elements: 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Each atom’s size is relative to the largest element, cesium. In this section, we discuss how atomic and ion “sizes” are defined and obtained. Although the concept. Smallest Atomic Size.
From izabella-well-wiggins.blogspot.com
How Do You Find the Smallest Atomic Radius Smallest Atomic Size The atomic radius is an indication of the size of an atom. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Atom size values are calculated from atomic radius data. It follows that the smallest atoms derive the right of the table as we. Smallest Atomic Size.
From ar.inspiredpencil.com
Periodic Table Atomic Size Smallest Atomic Size The atomic radius is an indication of the size of an atom. In this section, we discuss how atomic and ion “sizes” are defined and obtained. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. Although the concept of a definite radius of an. Smallest Atomic Size.
From www.out-class.org
Atomic Size Explained! OutClass Smallest Atomic Size Excluding the noble gases, the smaller atoms from the right hand side, i.e. The atomic radius is an indication of the size of an atom. 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. It follows that the smallest atoms derive the right of. Smallest Atomic Size.
From elchoroukhost.net
Periodic Table Class 11 Ncert Notes Elcho Table Smallest Atomic Size 119 rows the atomic radius of a chemical element is the distance from the center of the nucleus to the outermost shell of an electron. The atomic radius is an indication of the size of an atom. 119 rows access detailed info on all elements: Each atom’s size is relative to the largest element, cesium. This table shows how the. Smallest Atomic Size.
From www.slideserve.com
PPT Atomic Size PowerPoint Presentation, free download ID6875591 Smallest Atomic Size The atomic radius is an indication of the size of an atom. Although the concept of a definite radius of an atom is a bit fuzzy, atoms behave as if they have a certain radius. It follows that the smallest atoms derive the right of the table as we face it. 119 rows the atomic radius of a chemical element. Smallest Atomic Size.
From saylordotorg.github.io
The Periodic Table Smallest Atomic Size The atomic radius of the elements increases as we go from right to left across a period and as we go down the periods in a group. Excluding the noble gases, the smaller atoms from the right hand side, i.e. It follows that the smallest atoms derive the right of the table as we face it. In this section, we. Smallest Atomic Size.
From chem.libretexts.org
7.3 Sizes of Atoms and Ions Chemistry LibreTexts Smallest Atomic Size Atomic mass, electron configurations, charges, and more. Excluding the noble gases, the smaller atoms from the right hand side, i.e. 119 rows access detailed info on all elements: The atomic radius is an indication of the size of an atom. Each atom’s size is relative to the largest element, cesium. As a result, atoms and ions cannot be said to. Smallest Atomic Size.
From socratic.org
How to arrange the following atoms and ions in order of increasing Smallest Atomic Size In this section, we discuss how atomic and ion “sizes” are defined and obtained. It follows that the smallest atoms derive the right of the table as we face it. This table shows how the atom size, and atomic radius values change as you move horizontally and vertically across the periodic table. Excluding the noble gases, the smaller atoms from. Smallest Atomic Size.