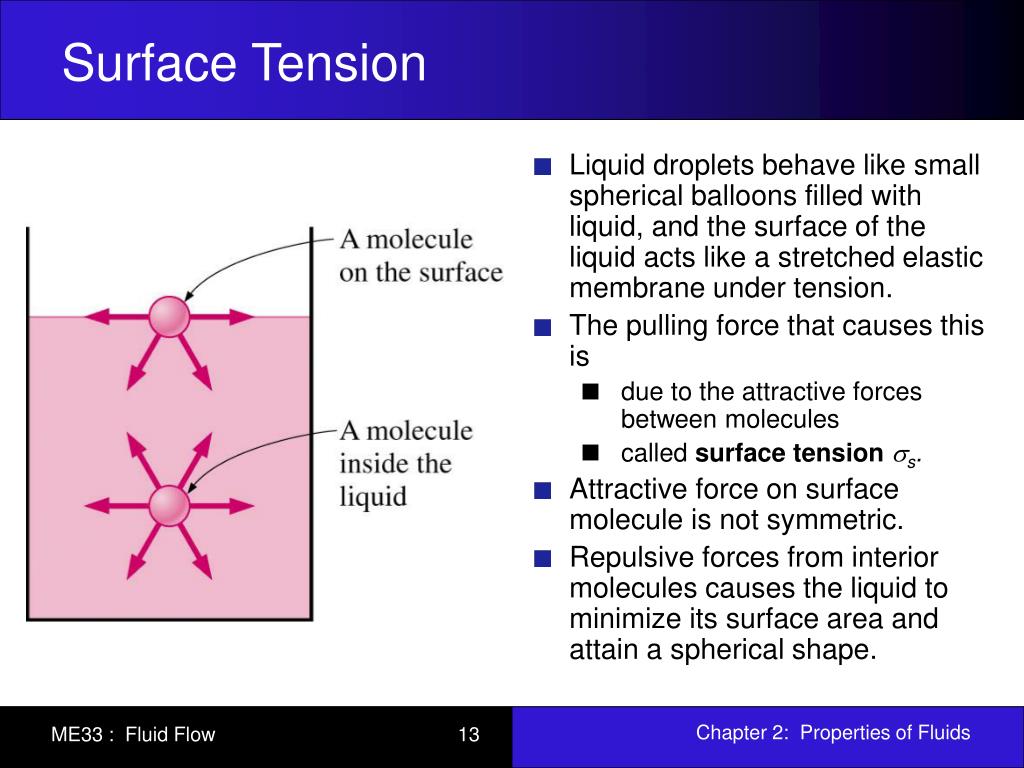
Surface Tension High Liquid . Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surfactants like detergent), each solution exhibits differing surface tension properties. As a result, the liquid surface forms a “skin” with minimum surface area and energy. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. This phenomenon can be observed in the nearly. Since these intermolecular forces vary depending on the nature of the liquid (e.g. The molecules on the surface of a liquid are attracted by their neighbors from the sides and bottom. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. A steel needle carefully placed on water will float. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking. This attraction of molecules towards one another is known as an intermolecular force. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Gasoline) or solutes in the liquid (e.g. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules.
from www.slideserve.com
Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result, the liquid surface forms a “skin” with minimum surface area and energy. Surfactants like detergent), each solution exhibits differing surface tension properties. Since these intermolecular forces vary depending on the nature of the liquid (e.g. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Gasoline) or solutes in the liquid (e.g.
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free
Surface Tension High Liquid Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. A steel needle carefully placed on water will float. This phenomenon can be observed in the nearly. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Surfactants like detergent), each solution exhibits differing surface tension properties. Gasoline) or solutes in the liquid (e.g. This attraction of molecules towards one another is known as an intermolecular force. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result, the liquid surface forms a “skin” with minimum surface area and energy. The molecules on the surface of a liquid are attracted by their neighbors from the sides and bottom.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID9168408 Surface Tension High Liquid “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Since these intermolecular forces vary depending on the nature of the. Surface Tension High Liquid.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry Surface Tension High Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension, property of a liquid surface displayed by its acting. Surface Tension High Liquid.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension High Liquid This phenomenon can be observed in the nearly. A steel needle carefully placed on water will float. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid. Surface Tension High Liquid.
From www.britannica.com
surface tension Definition, Examples, & Facts Surface Tension High Liquid This phenomenon can be observed in the nearly. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This attraction of molecules towards. Surface Tension High Liquid.
From www.youtube.com
Surface Tension on Liquid Jet formula derivation with 3d animation Surface Tension High Liquid A steel needle carefully placed on water will float. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand. Surface Tension High Liquid.
From www.slideserve.com
PPT Chapter 11 Liquids and Intermolecular Forces PowerPoint Surface Tension High Liquid Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. As a result, the liquid surface forms a “skin” with minimum surface area and energy. Simply put, surface tension is the tendency of molecules of a liquid. Surface Tension High Liquid.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension High Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Gasoline) or solutes in the liquid (e.g. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Among common liquids, water exhibits a distinctly. Surface Tension High Liquid.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Surface Tension High Liquid Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Since these intermolecular forces vary depending on the nature of the liquid (e.g. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking. Gasoline) or solutes in. Surface Tension High Liquid.
From www.dataphysics-instruments.com
Interfacial and surface tension of liquids explained DataPhysics Surface Tension High Liquid Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surfactants like detergent), each solution exhibits differing surface tension properties. This attraction of molecules towards one another is known as an. Surface Tension High Liquid.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b Surface Tension High Liquid Gasoline) or solutes in the liquid (e.g. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. As a result, the liquid surface forms a “skin” with minimum surface area and energy. This attraction of molecules towards one another is known as an intermolecular force. Among common liquids, water exhibits a. Surface Tension High Liquid.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID Surface Tension High Liquid A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. As a result of this high surface tension, the surface of water represents. Surface Tension High Liquid.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Surface Tension High Liquid This attraction of molecules towards one another is known as an intermolecular force. Surfactants like detergent), each solution exhibits differing surface tension properties. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can. Surface Tension High Liquid.
From ucscphysicsdemo.sites.ucsc.edu
Surface Tension of Liquids UCSC Physics Demonstration Room Surface Tension High Liquid Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. This attraction of molecules towards one another is known as an intermolecular force. This phenomenon can be observed in the nearly. Surfactants like detergent), each solution exhibits differing. Surface Tension High Liquid.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension High Liquid Surfactants like detergent), each solution exhibits differing surface tension properties. As a result, the liquid surface forms a “skin” with minimum surface area and energy. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking. A low surface tension can cause liquids to spread easily on. Surface Tension High Liquid.
From users.highland.edu
Unique Properties of Liquids Surface Tension High Liquid As a result, the liquid surface forms a “skin” with minimum surface area and energy. This attraction of molecules towards one another is known as an intermolecular force. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surfactants like detergent), each solution exhibits differing surface tension properties. “surface tension is. Surface Tension High Liquid.
From www.numerade.com
SOLVED Text Liquid A is known to have higher surface tension and Surface Tension High Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Gasoline) or solutes in the liquid (e.g. This phenomenon can be observed in the nearly. This attraction of molecules towards one another is known as an intermolecular force. As. Surface Tension High Liquid.
From www.slideserve.com
PPT Solids, Liquids, Gases (and Solutions) PowerPoint Presentation Surface Tension High Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the sides and bottom. This attraction of molecules towards one another is known as an intermolecular force. A low surface tension can cause liquids to spread easily on surfaces and form. Surface Tension High Liquid.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension High Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension, property of a liquid surface. Surface Tension High Liquid.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension High Liquid The molecules on the surface of a liquid are attracted by their neighbors from the sides and bottom. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. As a result of this high surface tension, the surface of water represents a relatively. Surface Tension High Liquid.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Surface Tension High Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. A steel needle carefully placed on water will float. Surfactants like detergent), each solution exhibits differing surface tension properties. A low surface tension can cause liquids to spread easily. Surface Tension High Liquid.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Surface Tension High Liquid This attraction of molecules towards one another is known as an intermolecular force. Surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to. Surface Tension High Liquid.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension High Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. This attraction of molecules towards one another is known as an intermolecular force. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. A. Surface Tension High Liquid.
From www.thoughtco.com
Surface Tension Definition in Chemistry Surface Tension High Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking. Surface tension, property of a. Surface Tension High Liquid.
From www.youtube.com
Why are liquid drops spherical? Surface Tension YouTube Surface Tension High Liquid Surfactants like detergent), each solution exhibits differing surface tension properties. This phenomenon can be observed in the nearly. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is the energy, or work, required to increase the. Surface Tension High Liquid.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension High Liquid A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. A steel needle carefully placed on water will float. Surfactants like detergent), each solution exhibits. Surface Tension High Liquid.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension High Liquid Surfactants like detergent), each solution exhibits differing surface tension properties. Among common liquids, water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. Gasoline) or solutes in the liquid (e.g. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. A steel needle carefully placed. Surface Tension High Liquid.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts Surface Tension High Liquid Since these intermolecular forces vary depending on the nature of the liquid (e.g. Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surface tension is the energy, or work, required to increase the surface area of a liquid. Surface Tension High Liquid.
From www.youtube.com
What is Surface Tension Liquid State Liquid Dynamics Surface Surface Tension High Liquid Surfactants like detergent), each solution exhibits differing surface tension properties. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a. Surface Tension High Liquid.
From www.vrogue.co
What Are The Importances Of Surface Tension And Visco vrogue.co Surface Tension High Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. Surfactants like detergent), each solution exhibits differing surface tension properties. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. This phenomenon can be. Surface Tension High Liquid.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Surface Tension High Liquid Gasoline) or solutes in the liquid (e.g. As a result, the liquid surface forms a “skin” with minimum surface area and energy. “surface tension is the tension of a liquid’s surface film caused by the bulk of the liquid’s attraction of the particles in the surface layer, which tends to minimize surface area.”. Since these intermolecular forces vary depending on. Surface Tension High Liquid.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Surface Tension High Liquid This phenomenon can be observed in the nearly. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. This attraction of molecules towards one another is known as an intermolecular force. Surface tension is the energy, or work, required to increase the. Surface Tension High Liquid.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension High Liquid As a result, the liquid surface forms a “skin” with minimum surface area and energy. A steel needle carefully placed on water will float. Since these intermolecular forces vary depending on the nature of the liquid (e.g. Gasoline) or solutes in the liquid (e.g. Surface tension is the energy, or work, required to increase the surface area of a liquid. Surface Tension High Liquid.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Surface Tension High Liquid Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. This phenomenon can be observed in the nearly. As a result of this high surface tension, the surface of water represents a relatively “tough skin” that can withstand considerable force without breaking. Surface tension is the energy, or work, required to increase the surface area. Surface Tension High Liquid.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension High Liquid Simply put, surface tension is the tendency of molecules of a liquid to be attracted more towards one another at the surface of a liquid than to the air above it. A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. As a. Surface Tension High Liquid.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free Surface Tension High Liquid A low surface tension can cause liquids to spread easily on surfaces and form an even coating, while a high surface tension can lead to uneven wetting. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Gasoline) or solutes in the liquid (e.g. Surface tension, property of a liquid. Surface Tension High Liquid.