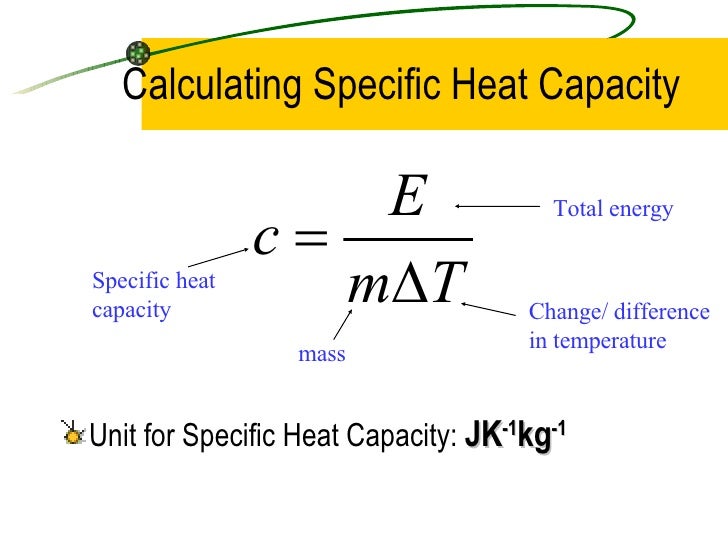
What Is Specific Heat Capacity Formula . Specific heat capacity may be reported in calories per gram degree celsius, too. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). In si units, specific heat capacity (symbol: The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It describes how much heat must be added to a unit of mass of a given substance to raise its. It may also be expressed as j/kg·k. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of a substance. Where q is the energy added and δt is the change in temperature. Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. This means that it takes 4,200 j to raise the.
from www.slideshare.net
Where q is the energy added and δt is the change in temperature. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. In si units, specific heat capacity (symbol: Specific heat capacity may be reported in calories per gram degree celsius, too. Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. This means that it takes 4,200 j to raise the. It may also be expressed as j/kg·k. We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of a substance.
Heat Capacity
What Is Specific Heat Capacity Formula Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. This means that it takes 4,200 j to raise the. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). In si units, specific heat capacity (symbol: We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. Specific heat capacity may be reported in calories per gram degree celsius, too. Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of a substance. Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. It describes how much heat must be added to a unit of mass of a given substance to raise its. Where q is the energy added and δt is the change in temperature. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It may also be expressed as j/kg·k.
From www.youtube.com
Specific Heat Capacity Short Example Solving for Mass YouTube What Is Specific Heat Capacity Formula The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). In si units, specific heat capacity (symbol: This means that it takes 4,200 j to raise the. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Specific heat capacity may. What Is Specific Heat Capacity Formula.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Specific Heat Capacity Formula Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. It describes how much heat must be added to a unit of mass of a given substance to raise its. This means that it takes 4,200 j to raise the. Unlike the total heat capacity, the specific heat. What Is Specific Heat Capacity Formula.
From oertx.highered.texas.gov
General Chemistry for Science Majors, Unit 1, Physical Properties What Is Specific Heat Capacity Formula This means that it takes 4,200 j to raise the. Specific heat capacity may be reported in calories per gram degree celsius, too. It describes how much heat must be added to a unit of mass of a given substance to raise its. C) is the amount of heat in joules required to raise 1 gram of a substance 1. What Is Specific Heat Capacity Formula.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula What Is Specific Heat Capacity Formula It may also be expressed as j/kg·k. In si units, specific heat capacity (symbol: Where q is the energy added and δt is the change in temperature. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. C) is the amount of heat in joules required to raise 1 gram of a substance 1. What Is Specific Heat Capacity Formula.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Specific Heat Capacity Formula Where q is the energy added and δt is the change in temperature. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It may also be expressed as j/kg·k. Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of. What Is Specific Heat Capacity Formula.
From www.markedbyteachers.com
Specific Heat Capacity International Baccalaureate Physics Marked What Is Specific Heat Capacity Formula Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of a substance. We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. Unlike the total heat capacity, the specific heat capacity is independent of mass or. What Is Specific Heat Capacity Formula.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii What Is Specific Heat Capacity Formula It describes how much heat must be added to a unit of mass of a given substance to raise its. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). The formula for specific heat capacity, c,. What Is Specific Heat Capacity Formula.
From testbook.com
Specific Heat Capacity Learning Notes for IIT JEE Testbook What Is Specific Heat Capacity Formula Where q is the energy added and δt is the change in temperature. In si units, specific heat capacity (symbol: Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). We can also use the specific heat equation to. What Is Specific Heat Capacity Formula.
From getrevising.co.uk
Physicsend of year 9 Revision Cards in GCSE Physics What Is Specific Heat Capacity Formula Where q is the energy added and δt is the change in temperature. It may also be expressed as j/kg·k. In si units, specific heat capacity (symbol: The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Specific heat capacity formula the definition of specific heat capacity of any. What Is Specific Heat Capacity Formula.
From www.youtube.com
Specific Heat Capacity Mr E Physics P3 AQA GCSE Physics YouTube What Is Specific Heat Capacity Formula The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. It may also be expressed as j/kg·k. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The. What Is Specific Heat Capacity Formula.
From www.youtube.com
Specific Heat Equation Stated Clearly YouTube What Is Specific Heat Capacity Formula C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. This means that it takes 4,200 j to raise the. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). It may also be expressed as j/kg·k. The formula for specific heat capacity, c, of a substance. What Is Specific Heat Capacity Formula.
From haipernews.com
How To Calculate Specific Heat Capacity Bbc Bitesize Haiper What Is Specific Heat Capacity Formula The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). In si units, specific heat capacity (symbol: Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of. What Is Specific Heat Capacity Formula.
From worksheetlistsog.z21.web.core.windows.net
Formula For Calculating Specific Heat What Is Specific Heat Capacity Formula Specific heat capacity may be reported in calories per gram degree celsius, too. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The formula for specific heat capacity, c, of a substance with mass m, is. What Is Specific Heat Capacity Formula.
From studyfinder.org
Learn Everything You Need to Know About Specific Heat Capacity in our What Is Specific Heat Capacity Formula We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. This means that it takes 4,200 j to raise the. Specific heat capacity may be reported in calories per gram degree celsius, too. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c).. What Is Specific Heat Capacity Formula.
From www.slideshare.net
Heat Capacity What Is Specific Heat Capacity Formula It describes how much heat must be added to a unit of mass of a given substance to raise its. It may also be expressed as j/kg·k. In si units, specific heat capacity (symbol: C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Unlike the total heat capacity, the specific. What Is Specific Heat Capacity Formula.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 What Is Specific Heat Capacity Formula Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. In si units, specific heat capacity (symbol: Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. The formula for specific heat capacity, c, of a substance with mass m, is c. What Is Specific Heat Capacity Formula.
From thechemistrynotes.com
Heat Capacity and Specific Heat What Is Specific Heat Capacity Formula It may also be expressed as j/kg·k. This means that it takes 4,200 j to raise the. Specific heat capacity may be reported in calories per gram degree celsius, too. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It describes how much heat must be added to a unit of. What Is Specific Heat Capacity Formula.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube What Is Specific Heat Capacity Formula Where q is the energy added and δt is the change in temperature. Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of a substance. In si units, specific heat capacity (symbol: It may also be expressed as j/kg·k. Specific heat capacity may be reported in calories. What Is Specific Heat Capacity Formula.
From quizzlibhofmann.z19.web.core.windows.net
Specific Heat Calculation Examples What Is Specific Heat Capacity Formula The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). In si units, specific heat capacity (symbol: It may also be expressed as j/kg·k. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The specific heat capacity of water is. What Is Specific Heat Capacity Formula.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Is Specific Heat Capacity Formula We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. Where q is the energy added and δt is the change in temperature. Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of a substance. The. What Is Specific Heat Capacity Formula.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Specific Heat Capacity Formula Specific heat capacity may be reported in calories per gram degree celsius, too. Specific heat and heat capacity formula the specific heat can be calculated from the amount of heat transferred into and out of a substance. It describes how much heat must be added to a unit of mass of a given substance to raise its. Specific heat capacity. What Is Specific Heat Capacity Formula.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video What Is Specific Heat Capacity Formula Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat capacity may be reported in calories per gram degree celsius, too. We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. In si units, specific heat capacity (symbol: Where q. What Is Specific Heat Capacity Formula.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free What Is Specific Heat Capacity Formula The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). It may also be expressed as j/kg·k. Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. The specific heat capacity of water is 4,200 joules per kilogram. What Is Specific Heat Capacity Formula.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Specific Heat Capacity Formula The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). In si units, specific heat capacity (symbol: It describes how much heat must be added to a unit of mass of a given substance to raise its. Specific heat and heat capacity formula the specific heat can be calculated. What Is Specific Heat Capacity Formula.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube What Is Specific Heat Capacity Formula It describes how much heat must be added to a unit of mass of a given substance to raise its. This means that it takes 4,200 j to raise the. Where q is the energy added and δt is the change in temperature. The formula for specific heat capacity, c, of a substance with mass m, is c = q. What Is Specific Heat Capacity Formula.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is Specific Heat Capacity Formula Where q is the energy added and δt is the change in temperature. Specific heat capacity may be reported in calories per gram degree celsius, too. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The formula for specific heat capacity, c, of a substance with mass m, is c =. What Is Specific Heat Capacity Formula.
From www.grc.nasa.gov
Specific Heats What Is Specific Heat Capacity Formula Specific heat capacity may be reported in calories per gram degree celsius, too. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). The formula for specific heat capacity, c, of a substance with mass m, is c = q. What Is Specific Heat Capacity Formula.
From studylib.net
Heat Equation What Is Specific Heat Capacity Formula Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. It describes how much heat. What Is Specific Heat Capacity Formula.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems What Is Specific Heat Capacity Formula We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Unlike the total heat capacity,. What Is Specific Heat Capacity Formula.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Specific Heat Capacity Formula We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. In si units, specific heat capacity (symbol: The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The specific heat capacity of water is 4,200 joules per. What Is Specific Heat Capacity Formula.
From www.youtube.com
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial What Is Specific Heat Capacity Formula Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. It may also be expressed as j/kg·k. We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. Specific heat capacity may be reported in calories per. What Is Specific Heat Capacity Formula.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Is Specific Heat Capacity Formula Specific heat capacity may be reported in calories per gram degree celsius, too. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). It describes how much heat must be added to a unit of mass of a given substance to raise its. The specific heat capacity of water is. What Is Specific Heat Capacity Formula.
From gcsephysicsninja.com
35. Specific heat capacity equation What Is Specific Heat Capacity Formula Specific heat capacity may be reported in calories per gram degree celsius, too. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Specific heat capacity formula the definition of specific heat capacity of any substance is “the quantity of heat required to change the. This means that it takes 4,200 j to raise the.. What Is Specific Heat Capacity Formula.
From slidetodoc.com
SPECIFIC HEAT Calculations SPECIFIC HEAT CAPACITY The amount What Is Specific Heat Capacity Formula Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). It may also be expressed as j/kg·k. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Specific heat and. What Is Specific Heat Capacity Formula.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is Specific Heat Capacity Formula C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It describes how much heat must be added to a unit of mass of a given substance to raise its. We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity.. What Is Specific Heat Capacity Formula.