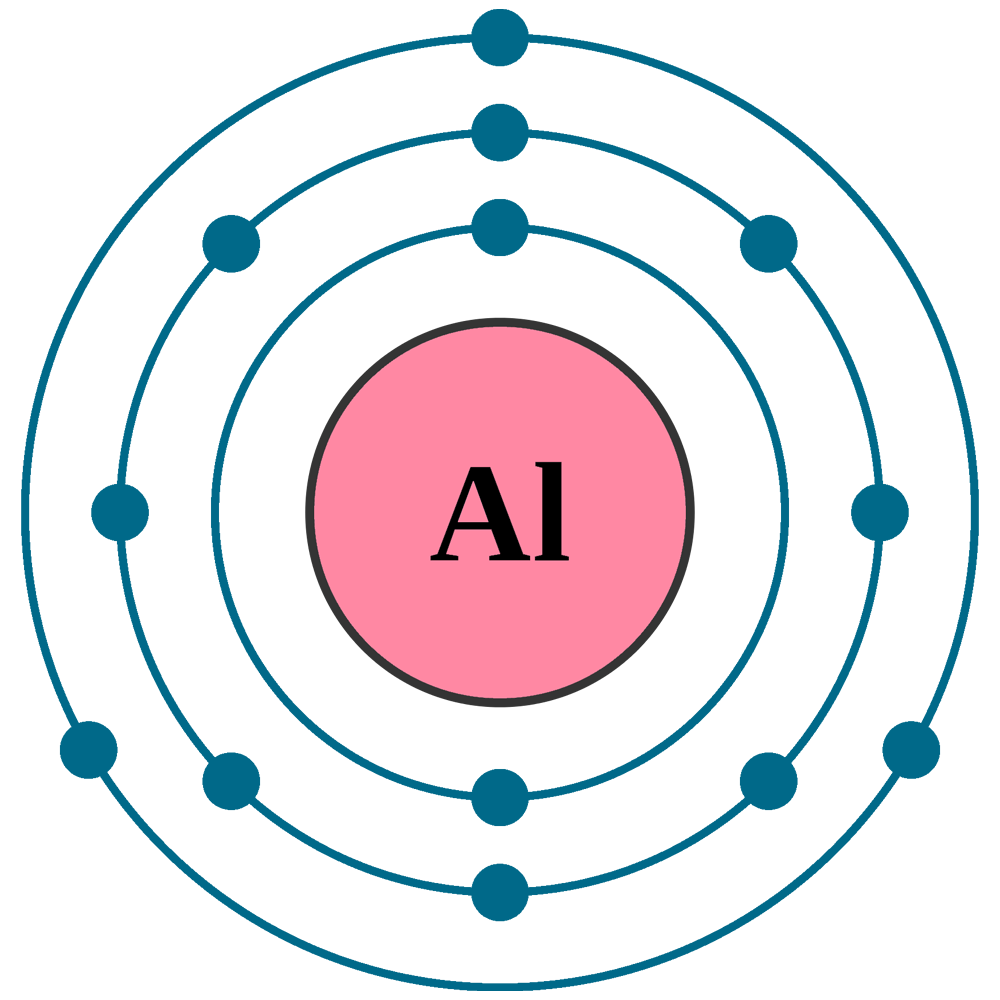
Aluminum Number Of Electrons . Aluminium has 13 electrons and 14 neutrons. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Hence the aluminum element has electrons arrangement 2, 8, 3. This electron arrangement indicates that the outermost. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. The proton number is also 13. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. Aluminium has 3 electrons in valence shell [3] [6]. Aluminum has 13 electrons, 13. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Three oxidation states of aluminium are +3, +1 and+2. Now the atomic number of aluminum (al) is 13.
from elchoroukhost.net
Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Hence the aluminum element has electrons arrangement 2, 8, 3. This electron arrangement indicates that the outermost. Aluminium has 13 electrons and 14 neutrons. Three oxidation states of aluminium are +3, +1 and+2. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Aluminium has 3 electrons in valence shell [3] [6]. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart.
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table
Aluminum Number Of Electrons This electron arrangement indicates that the outermost. Now the atomic number of aluminum (al) is 13. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Three oxidation states of aluminium are +3, +1 and+2. Hence the aluminum element has electrons arrangement 2, 8, 3. The proton number is also 13. Aluminium has 13 electrons and 14 neutrons. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. Aluminum has 13 electrons, 13. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. This electron arrangement indicates that the outermost. Aluminium has 3 electrons in valence shell [3] [6].
From www.alamy.com
Symbol and electron diagram Aluminium Stock Vector Image & Art Alamy Aluminum Number Of Electrons The proton number is also 13. This electron arrangement indicates that the outermost. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Hence the aluminum element has electrons arrangement 2, 8, 3. Aluminium has 13 electrons and 14 neutrons. Aluminium has 3 electrons in valence shell [3] [6]. The electron configuration for aluminum. Aluminum Number Of Electrons.
From www.youtube.com
Al 3+ Electron Configuration (Aluminum Ion) YouTube Aluminum Number Of Electrons Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Aluminum has 13 electrons, 13. Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium.. Aluminum Number Of Electrons.
From www.alamy.com
A Aluminium atom diagram Stock Vector Image & Art Alamy Aluminum Number Of Electrons Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. Aluminium has 13 electrons and 14 neutrons. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. This electron arrangement indicates that the outermost. Now the atomic number of aluminum (al) is 13. Learn how to write the electron configuration. Aluminum Number Of Electrons.
From www.museoinclusivo.com
Exploring Aluminum Protons Neutrons Electrons An Indepth Look Aluminum Number Of Electrons Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Now the atomic number of aluminum. Aluminum Number Of Electrons.
From www.museoinclusivo.com
Exploring the Impact of Aluminum’s Number of Electrons on Its Physical Aluminum Number Of Electrons Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Now the atomic number of aluminum (al) is 13. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s. Aluminum Number Of Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Number Of Electrons The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Three oxidation states of aluminium are +3, +1 and+2. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Now the atomic number of aluminum (al) is 13. Aluminium has 3 electrons in valence shell. Aluminum Number Of Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Number Of Electrons Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Aluminium has 13 electrons and 14 neutrons. The proton number is also 13. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. This electron arrangement indicates that the outermost. Aluminium has 3 electrons in valence shell [3]. Aluminum Number Of Electrons.
From favpng.com
Aluminium Electron Shell Electron Configuration Chemical Element Atom Aluminum Number Of Electrons Aluminum has 13 electrons, 13. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Aluminium has 13 electrons and 14 neutrons. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Find out the stable and unstable isotopes, oxidation states and. Aluminum Number Of Electrons.
From www.museoinclusivo.com
How Many Electrons Does an Aluminum Atom Have? Exploring the Atomic Aluminum Number Of Electrons Three oxidation states of aluminium are +3, +1 and+2. Now the atomic number of aluminum (al) is 13. Aluminium has 13 electrons and 14 neutrons. Hence the aluminum element has electrons arrangement 2, 8, 3. This electron arrangement indicates that the outermost. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13. Aluminum Number Of Electrons.
From www.vectorstock.com
Diagram representation of the element aluminium Vector Image Aluminum Number Of Electrons The proton number is also 13. Aluminum has 13 electrons, 13. Aluminium has 13 electrons and 14 neutrons. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. The. Aluminum Number Of Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminum Number Of Electrons Three oxidation states of aluminium are +3, +1 and+2. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Hence the aluminum element has electrons arrangement 2, 8, 3. Now the atomic number of aluminum (al) is 13. Learn about the chemical. Aluminum Number Of Electrons.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Aluminum Number Of Electrons Aluminium has 13 electrons and 14 neutrons. Aluminum has 13 electrons, 13. Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. In compound al 2 o 3, aluminium has oxidationstate of +3 [4].. Aluminum Number Of Electrons.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Aluminum Number Of Electrons Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Hence the aluminum element has electrons arrangement 2, 8, 3. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Aluminum has 13 electrons, 13. This electron arrangement indicates that the outermost.. Aluminum Number Of Electrons.
From www.museoinclusivo.com
The Number of Electrons in Aluminum An Exploration Aluminum Profile Blog Aluminum Number Of Electrons Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Aluminium has 13 electrons and 14 neutrons. Aluminium has 3 electrons in valence shell [3] [6]. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. This electron arrangement indicates that the. Aluminum Number Of Electrons.
From periodictable.me
How Can We Find Electron Configuration For Aluminium (Al) Aluminum Number Of Electrons This electron arrangement indicates that the outermost. Aluminium has 13 electrons and 14 neutrons. Hence the aluminum element has electrons arrangement 2, 8, 3. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Aluminum has 13 electrons, 13. Aluminum is the 13th element in the periodic table and has an. Aluminum Number Of Electrons.
From ar.inspiredpencil.com
Aluminium Atomic Structure Aluminum Number Of Electrons In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. The proton number is also 13. This electron arrangement indicates that the outermost. Aluminium has 3 electrons in valence shell [3] [6]. Aluminium has 13 electrons and 14 neutrons. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Aluminum is the. Aluminum Number Of Electrons.
From www.dreamstime.com
Model of aluminium atom stock vector. Illustration of science 164474877 Aluminum Number Of Electrons Now the atomic number of aluminum (al) is 13. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Aluminum has 13 electrons, 13. Three oxidation states of aluminium are +3, +1 and+2. The electron configuration for aluminum is. Aluminum Number Of Electrons.
From sciencenotes.org
Aluminum Atom Science Notes and Projects Aluminum Number Of Electrons This electron arrangement indicates that the outermost. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Aluminum has 13 electrons, 13. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Hence. Aluminum Number Of Electrons.
From topblogtenz.com
Aluminum Orbital diagram, Electron configuration, and Valence electrons Aluminum Number Of Electrons Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. The proton number is also 13. Three oxidation states of aluminium are +3, +1 and+2. Aluminium has 13 electrons and 14 neutrons. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Now the atomic number of aluminum (al) is. Aluminum Number Of Electrons.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Aluminum Number Of Electrons Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Aluminium has 3 electrons in valence shell [3] [6]. Learn how to. Aluminum Number Of Electrons.
From periodictableguide.com
Aluminum (Al) Periodic Table (Element Information & More) Aluminum Number Of Electrons Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Aluminium has 13 electrons and 14 neutrons. This electron arrangement indicates that the outermost. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth.. Aluminum Number Of Electrons.
From www.sciencephoto.com
Aluminum, atomic structure Stock Image C018/3694 Science Photo Library Aluminum Number Of Electrons The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. The proton number is also 13. Hence the. Aluminum Number Of Electrons.
From www.museoinclusivo.com
Exploring the Number of Electrons in Aluminum Aluminum Profile Blog Aluminum Number Of Electrons Aluminum has 13 electrons, 13. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. This electron arrangement indicates that the outermost. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Learn how to write the electron configuration for aluminium (al). Aluminum Number Of Electrons.
From periodictable.me
Aluminum Valence Electrons Aluminum Valency (Al) with Dot Diagram Aluminum Number Of Electrons The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Hence the aluminum element has electrons arrangement 2, 8, 3. Now the atomic number of aluminum (al) is 13. This electron arrangement indicates that the outermost. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. In compound. Aluminum Number Of Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Number Of Electrons Aluminum has 13 electrons, 13. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Now the atomic number of aluminum (al) is 13. The electron configuration for aluminum is 1s 2. Aluminum Number Of Electrons.
From ar.inspiredpencil.com
Aluminium Electron Configuration Aluminum Number Of Electrons Aluminum has 13 electrons, 13. Aluminium has 3 electrons in valence shell [3] [6]. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Aluminium has 13 electrons and 14 neutrons. Now the atomic number. Aluminum Number Of Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Number Of Electrons Aluminium has 3 electrons in valence shell [3] [6]. This electron arrangement indicates that the outermost. Aluminum has 13 electrons, 13. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Hence the aluminum element has electrons arrangement. Aluminum Number Of Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Al Aluminium Element Information Facts, Properties, Trends, Uses and Aluminum Number Of Electrons In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Hence the aluminum element has electrons arrangement 2, 8, 3. Aluminum is the 13th element in the periodic table and has an atomic number of 13 and 13 electrons in three shells. Learn how to write the electron configuration for aluminium (al) using the period table or an. Aluminum Number Of Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Number Of Electrons Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Aluminum has 13 electrons, 13. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Aluminium has 13 electrons and 14 neutrons. Hence the aluminum element has electrons arrangement 2, 8, 3. The proton number. Aluminum Number Of Electrons.
From www.animalia-life.club
Aluminium Periodic Table Symbol Aluminum Number Of Electrons Aluminium has 3 electrons in valence shell [3] [6]. This electron arrangement indicates that the outermost. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Aluminium has 13 electrons and 14 neutrons. Three oxidation states of aluminium are +3, +1 and+2. Aluminum has 13 electrons, 13. Learn about the properties, history, compounds and reactions of aluminum, the. Aluminum Number Of Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Aluminum (Al) YouTube Aluminum Number Of Electrons Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. Hence the aluminum element has electrons arrangement 2, 8,. Aluminum Number Of Electrons.
From www.alamy.com
Atom model aluminium Stock Vector Images Alamy Aluminum Number Of Electrons Three oxidation states of aluminium are +3, +1 and+2. The proton number is also 13. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Learn about the properties, history, compounds and reactions of aluminum, the most abundant metal on earth. The. Aluminum Number Of Electrons.
From valenceelectrons.com
How to Write the Electron Configuration for Aluminium (Al)? Aluminum Number Of Electrons The proton number is also 13. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. Three oxidation states of aluminium are +3, +1 and+2. The electron configuration for aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Aluminum has 13 electrons, 13. Aluminum is the 13th element in the periodic table and has an. Aluminum Number Of Electrons.
From www.pngitem.com
Electron Shell 013 Aluminium Aluminum Electron Shell Diagram, HD Png Aluminum Number Of Electrons Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. The proton number is also 13. In compound al 2 o 3, aluminium has oxidationstate of +3 [4]. This electron arrangement indicates that the outermost. Find out the stable and unstable isotopes, oxidation states and common alloys of aluminium. Aluminium has 3 electrons in. Aluminum Number Of Electrons.
From elchoroukhost.net
Aluminum Periodic Table Protons Neutrons Electrons Elcho Table Aluminum Number Of Electrons Learn how to write the electron configuration for aluminium (al) using the period table or an electron configuration chart. Now the atomic number of aluminum (al) is 13. Learn about the chemical and physical properties of aluminum, the 13th element of the periodic table. The proton number is also 13. Find out the stable and unstable isotopes, oxidation states and. Aluminum Number Of Electrons.