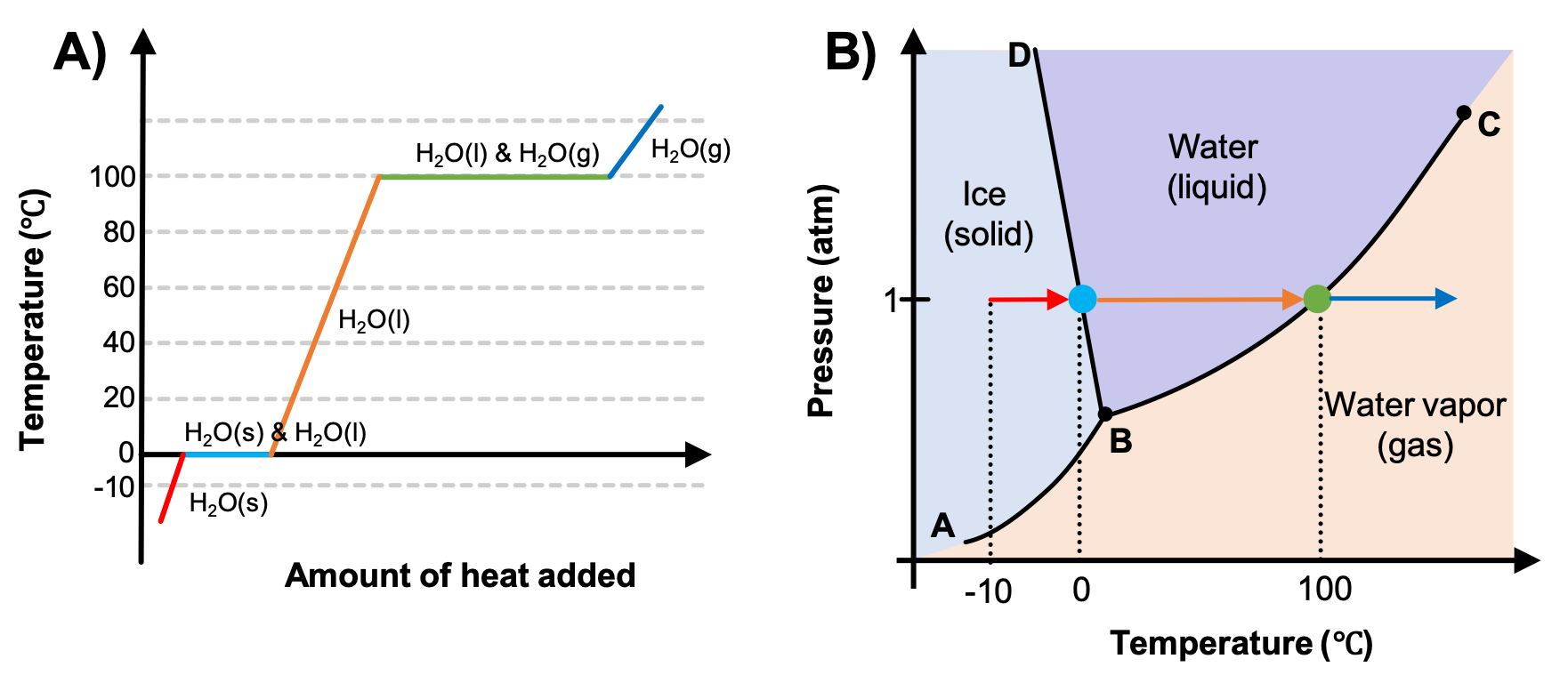
When Water Is Heated In A Vessel After Some Time . Enter the mass, initial and final temperature, and specific heat. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The phenomenon of superheating causes “bumping” when a liquid is heated in the. When water is heated with an immersion heater, one first observes a rise in temperature. Superheated liquids are not stable; Calculate the energy and time needed to heat water from one state to another. This essentially means that we started with a. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. Given sufficient heat, the vessel will burst. I came across a question where water was being heated in a calorimetry whose water equivalent was given. But during vaporization, the temperature does not increase any further. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. The liquid will eventually boil, sometimes violently.
from wisc.pb.unizin.org
Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. But during vaporization, the temperature does not increase any further. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. Given sufficient heat, the vessel will burst. When water is heated with an immersion heater, one first observes a rise in temperature. Enter the mass, initial and final temperature, and specific heat. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Calculate the energy and time needed to heat water from one state to another. Superheated liquids are not stable; This essentially means that we started with a.
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book
When Water Is Heated In A Vessel After Some Time This essentially means that we started with a. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Given sufficient heat, the vessel will burst. The liquid will eventually boil, sometimes violently. Calculate the energy and time needed to heat water from one state to another. Superheated liquids are not stable; This essentially means that we started with a. When water is heated with an immersion heater, one first observes a rise in temperature. The phenomenon of superheating causes “bumping” when a liquid is heated in the. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. Enter the mass, initial and final temperature, and specific heat. But during vaporization, the temperature does not increase any further. I came across a question where water was being heated in a calorimetry whose water equivalent was given. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an.
From www.numerade.com
The diagram shows how a beaker of water is heated by convection when it When Water Is Heated In A Vessel After Some Time This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The liquid will eventually boil, sometimes violently. This essentially means that we started with a. I came across a question where water was being heated in a calorimetry whose water equivalent was. When Water Is Heated In A Vessel After Some Time.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts When Water Is Heated In A Vessel After Some Time Superheated liquids are not stable; The phenomenon of superheating causes “bumping” when a liquid is heated in the. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Calculate the energy and time needed to heat water from one state to another.. When Water Is Heated In A Vessel After Some Time.
From askfilo.com
WATER CHANGES ITS FORM What happens when water is heated? What comes out When Water Is Heated In A Vessel After Some Time But during vaporization, the temperature does not increase any further. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. The liquid will eventually boil, sometimes violently. The phenomenon of superheating causes “bumping” when a liquid is heated in the. Given sufficient heat, the vessel will burst. Calculate the energy and time. When Water Is Heated In A Vessel After Some Time.
From www.sciencephoto.com
Convection current in heated water, illustration Stock Image C050 When Water Is Heated In A Vessel After Some Time But during vaporization, the temperature does not increase any further. When water is heated with an immersion heater, one first observes a rise in temperature. This essentially means that we started with a. Enter the mass, initial and final temperature, and specific heat. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an.. When Water Is Heated In A Vessel After Some Time.
From www.coursehero.com
[Solved] . 7 A rigid 10—L vessel initially contains a mixture of When Water Is Heated In A Vessel After Some Time The phenomenon of superheating causes “bumping” when a liquid is heated in the. I came across a question where water was being heated in a calorimetry whose water equivalent was given. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. Calculate the energy and time needed to heat water from one. When Water Is Heated In A Vessel After Some Time.
From www.snexplores.org
Explainer What are the different states of matter? When Water Is Heated In A Vessel After Some Time This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Superheated liquids are not stable; When water is heated with an immersion heater, one first observes a rise in temperature. Enter the mass, initial and final temperature, and specific heat. I came. When Water Is Heated In A Vessel After Some Time.
From idronics.caleffi.com
2 HEAT EXCHANGER TYPES Caleffi Idronics When Water Is Heated In A Vessel After Some Time But during vaporization, the temperature does not increase any further. The liquid will eventually boil, sometimes violently. Given sufficient heat, the vessel will burst. Superheated liquids are not stable; If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. Enter the mass, initial and final temperature, and specific heat. This essentially means. When Water Is Heated In A Vessel After Some Time.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk When Water Is Heated In A Vessel After Some Time When water is heated with an immersion heater, one first observes a rise in temperature. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an.. When Water Is Heated In A Vessel After Some Time.
From www.alamy.com
convection currents in a beaker of water heated by bunsen burner shown When Water Is Heated In A Vessel After Some Time Given sufficient heat, the vessel will burst. The liquid will eventually boil, sometimes violently. Superheated liquids are not stable; When water is heated with an immersion heater, one first observes a rise in temperature. I came across a question where water was being heated in a calorimetry whose water equivalent was given. But during vaporization, the temperature does not increase. When Water Is Heated In A Vessel After Some Time.
From ch301.cm.utexas.edu
heating curve When Water Is Heated In A Vessel After Some Time Given sufficient heat, the vessel will burst. But during vaporization, the temperature does not increase any further. This essentially means that we started with a. The phenomenon of superheating causes “bumping” when a liquid is heated in the. The liquid will eventually boil, sometimes violently. I came across a question where water was being heated in a calorimetry whose water. When Water Is Heated In A Vessel After Some Time.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet When Water Is Heated In A Vessel After Some Time Enter the mass, initial and final temperature, and specific heat. Given sufficient heat, the vessel will burst. Superheated liquids are not stable; I came across a question where water was being heated in a calorimetry whose water equivalent was given. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c. When Water Is Heated In A Vessel After Some Time.
From www.chegg.com
Solved 431 A rigid 10L vessel initially contains a mixture When Water Is Heated In A Vessel After Some Time Superheated liquids are not stable; Enter the mass, initial and final temperature, and specific heat. The phenomenon of superheating causes “bumping” when a liquid is heated in the. But during vaporization, the temperature does not increase any further. Calculate the energy and time needed to heat water from one state to another. Given sufficient heat, the vessel will burst. The. When Water Is Heated In A Vessel After Some Time.
From wisc.pb.unizin.org
M11Q2 Heating Curves and Phase Diagrams Chem 103/104 Resource Book When Water Is Heated In A Vessel After Some Time If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. The liquid will eventually boil, sometimes violently. Calculate the energy and time needed to heat water from one state to another. Enter the mass, initial and final temperature, and specific heat. This essentially means that we started with a. The phenomenon of. When Water Is Heated In A Vessel After Some Time.
From www.researchgate.net
Overview of the closed vessel heating test apparatus. Download When Water Is Heated In A Vessel After Some Time Given sufficient heat, the vessel will burst. I came across a question where water was being heated in a calorimetry whose water equivalent was given. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. Enter the mass, initial and final temperature, and specific heat. This plot of temperature shows what happens to. When Water Is Heated In A Vessel After Some Time.
From www.physics.brocku.ca
PPLATO FLAP PHYS 7.4 Specific heat, latent heat and entropy When Water Is Heated In A Vessel After Some Time Superheated liquids are not stable; But during vaporization, the temperature does not increase any further. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. The liquid will eventually boil, sometimes violently. This essentially means that we started with a. If we keep heating the mixture, after some time state 2 will lie. When Water Is Heated In A Vessel After Some Time.
From www.youtube.com
Fluid Mechanics Closed Vessel Contains Water w Air Pressure of 10 psi When Water Is Heated In A Vessel After Some Time When water is heated with an immersion heater, one first observes a rise in temperature. This essentially means that we started with a. The liquid will eventually boil, sometimes violently. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. Enter the mass, initial and final temperature, and specific heat. Calculate the energy. When Water Is Heated In A Vessel After Some Time.
From www.chegg.com
Solved A rigid 10L vessel initially contains a mixture of When Water Is Heated In A Vessel After Some Time Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Superheated liquids are not stable; The phenomenon of superheating causes “bumping” when a liquid is. When Water Is Heated In A Vessel After Some Time.
From www.chegg.com
Solved The two beakers below each have added to them the When Water Is Heated In A Vessel After Some Time I came across a question where water was being heated in a calorimetry whose water equivalent was given. The liquid will eventually boil, sometimes violently. Superheated liquids are not stable; This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Above ~650. When Water Is Heated In A Vessel After Some Time.
From arenahanna.wordpress.com
Transfer processing of heat energy HEAT WORLD OF PHYSICS When Water Is Heated In A Vessel After Some Time The phenomenon of superheating causes “bumping” when a liquid is heated in the. Given sufficient heat, the vessel will burst. But during vaporization, the temperature does not increase any further. When water is heated with an immersion heater, one first observes a rise in temperature. Enter the mass, initial and final temperature, and specific heat. Superheated liquids are not stable;. When Water Is Heated In A Vessel After Some Time.
From www.sciencephoto.com
Water boiling heated by bunsen burner Stock Image A300/0055 When Water Is Heated In A Vessel After Some Time Given sufficient heat, the vessel will burst. Calculate the energy and time needed to heat water from one state to another. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. Superheated liquids are not stable; Enter the mass, initial and final temperature, and specific heat. This plot of temperature shows what. When Water Is Heated In A Vessel After Some Time.
From www.tessshebaylo.com
Water Heating Equation Tessshebaylo When Water Is Heated In A Vessel After Some Time This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The phenomenon of superheating causes “bumping” when a liquid is heated in the. The liquid will eventually boil, sometimes violently. If we keep heating the mixture, after some time state 2 will. When Water Is Heated In A Vessel After Some Time.
From www.alamy.com
convection currents in a beaker of water being heated by a burning When Water Is Heated In A Vessel After Some Time When water is heated with an immersion heater, one first observes a rise in temperature. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: I came across a question where water was being heated in a calorimetry whose water equivalent was. When Water Is Heated In A Vessel After Some Time.
From slideplayer.com
Chemical Interactions ppt download When Water Is Heated In A Vessel After Some Time Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. I came across a question where water was being heated in a calorimetry whose water equivalent was given. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. When Water Is Heated In A Vessel After Some Time.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State When Water Is Heated In A Vessel After Some Time Given sufficient heat, the vessel will burst. Calculate the energy and time needed to heat water from one state to another. I came across a question where water was being heated in a calorimetry whose water equivalent was given. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. Superheated liquids are. When Water Is Heated In A Vessel After Some Time.
From www.w3schools.blog
Thermal Expansion of Liquids W3schools When Water Is Heated In A Vessel After Some Time Given sufficient heat, the vessel will burst. But during vaporization, the temperature does not increase any further. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The liquid will eventually boil, sometimes violently. Enter the mass, initial and final temperature, and. When Water Is Heated In A Vessel After Some Time.
From www.chegg.com
Solved You want to use an electric stove to heat some water. When Water Is Heated In A Vessel After Some Time Given sufficient heat, the vessel will burst. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The phenomenon of superheating causes “bumping” when a liquid is heated in the. The liquid will eventually boil, sometimes violently. This essentially means that we. When Water Is Heated In A Vessel After Some Time.
From aitchaeriesh.blogspot.com
33+ calculate heat absorbed by water AitchAeriesh When Water Is Heated In A Vessel After Some Time I came across a question where water was being heated in a calorimetry whose water equivalent was given. The phenomenon of superheating causes “bumping” when a liquid is heated in the. Calculate the energy and time needed to heat water from one state to another. Enter the mass, initial and final temperature, and specific heat. Above ~650 k, the water. When Water Is Heated In A Vessel After Some Time.
From www.sciencephoto.com
Water being heated Stock Image C009/8953 Science Photo Library When Water Is Heated In A Vessel After Some Time I came across a question where water was being heated in a calorimetry whose water equivalent was given. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at. When Water Is Heated In A Vessel After Some Time.
From sarahpetersonchemistry.blogspot.com
Summer Chemistry 2011 Activity 1 When Water Is Heated In A Vessel After Some Time Enter the mass, initial and final temperature, and specific heat. I came across a question where water was being heated in a calorimetry whose water equivalent was given. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. If we keep heating the mixture, after some time state 2 will lie on the. When Water Is Heated In A Vessel After Some Time.
From www.coursehero.com
[Solved] . 7 A rigid 10—L vessel initially contains a mixture of When Water Is Heated In A Vessel After Some Time Enter the mass, initial and final temperature, and specific heat. When water is heated with an immersion heater, one first observes a rise in temperature. Given sufficient heat, the vessel will burst. This essentially means that we started with a. Above ~650 k, the water can no longer be liquefied by pressure, and behaves more like an. This plot of. When Water Is Heated In A Vessel After Some Time.
From www.chegg.com
Solved Use the References to access important values if When Water Is Heated In A Vessel After Some Time This essentially means that we started with a. Enter the mass, initial and final temperature, and specific heat. I came across a question where water was being heated in a calorimetry whose water equivalent was given. Given sufficient heat, the vessel will burst. When water is heated with an immersion heater, one first observes a rise in temperature. Superheated liquids. When Water Is Heated In A Vessel After Some Time.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs When Water Is Heated In A Vessel After Some Time But during vaporization, the temperature does not increase any further. This essentially means that we started with a. I came across a question where water was being heated in a calorimetry whose water equivalent was given. Superheated liquids are not stable; When water is heated with an immersion heater, one first observes a rise in temperature. Enter the mass, initial. When Water Is Heated In A Vessel After Some Time.
From www.youtube.com
What Happens When Boiling Heat + Aluminium Vessel + Water Slo Mo When Water Is Heated In A Vessel After Some Time Calculate the energy and time needed to heat water from one state to another. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Given sufficient heat, the vessel will burst. I came across a question where water was being heated in. When Water Is Heated In A Vessel After Some Time.
From askfilo.com
A vessel containing water is heated from the top by means of a heater, ju.. When Water Is Heated In A Vessel After Some Time This essentially means that we started with a. But during vaporization, the temperature does not increase any further. I came across a question where water was being heated in a calorimetry whose water equivalent was given. Superheated liquids are not stable; The phenomenon of superheating causes “bumping” when a liquid is heated in the. Calculate the energy and time needed. When Water Is Heated In A Vessel After Some Time.
From www.chegg.com
Solved The closed vessel of the figure below contains water When Water Is Heated In A Vessel After Some Time But during vaporization, the temperature does not increase any further. Superheated liquids are not stable; When water is heated with an immersion heater, one first observes a rise in temperature. Given sufficient heat, the vessel will burst. If we keep heating the mixture, after some time state 2 will lie on the saturated vapor line. The liquid will eventually boil,. When Water Is Heated In A Vessel After Some Time.