Dilution Factor Using Concentration . Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Since the number of moles of solute must remain constant, the only way to achieve the. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. The purpose of a dilution is to decrease the concentration of the solution. Find out how to avoid common mistakes, understand. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn the formula, components, and applications of dilution factor in scientific research.
from www.slideshare.net
The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn the formula, components, and applications of dilution factor in scientific research. Find out how to avoid common mistakes, understand. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Since the number of moles of solute must remain constant, the only way to achieve the. The purpose of a dilution is to decrease the concentration of the solution. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution.
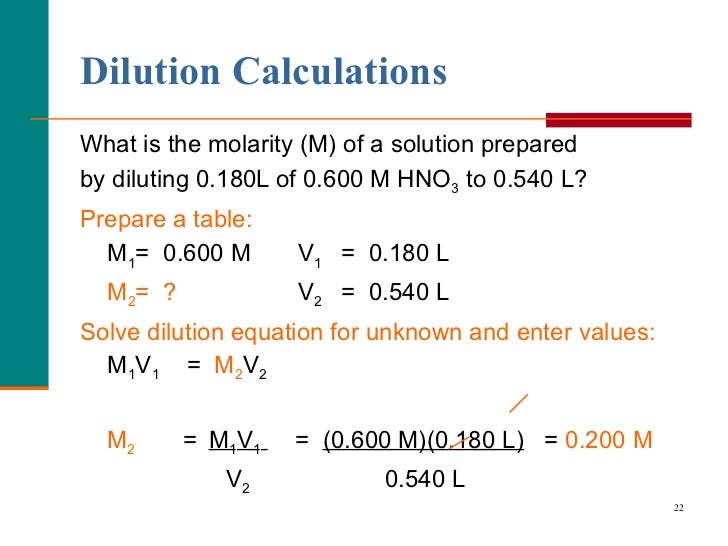
Molarity and dilution
Dilution Factor Using Concentration Learn the formula, components, and applications of dilution factor in scientific research. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Find out how to avoid common mistakes, understand. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. The purpose of a dilution is to decrease the concentration of the solution. Learn the formula, components, and applications of dilution factor in scientific research. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Since the number of moles of solute must remain constant, the only way to achieve the.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Factor Using Concentration Learn the formula, components, and applications of dilution factor in scientific research. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Learn how to use the dilution equation m1v1. Dilution Factor Using Concentration.
From support.axionbio.com
Understanding of Cell Sample Dilution Factor Dilution Factor Using Concentration Find out how to avoid common mistakes, understand. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Since the number of moles of solute must remain constant, the only way. Dilution Factor Using Concentration.
From www.coursehero.com
[Solved] Exercise 3 Concentration, Solution, and Dilution Data Table 8 Dilution Factor Using Concentration Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Since the number of moles of solute must remain constant, the only way to achieve the. The purpose of a dilution is to decrease the concentration of the solution. The dilution factor (or dilution ratio) is the notation used. Dilution Factor Using Concentration.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Factor Using Concentration Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn the formula, components, and applications of dilution factor in scientific research. Since the number of moles of solute must remain constant, the only way to achieve the. The dilution factor (or dilution ratio) is the notation used to. Dilution Factor Using Concentration.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Factor Using Concentration Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Find out how to avoid common mistakes, understand. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn the formula, components,. Dilution Factor Using Concentration.
From www.youtube.com
Calculate dilution factor and concentration when diluting liquid and Dilution Factor Using Concentration The purpose of a dilution is to decrease the concentration of the solution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. Dilution Factor Using Concentration.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Factor Using Concentration Find out how to avoid common mistakes, understand. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn the formula, components, and applications. Dilution Factor Using Concentration.
From printablezoneunglad.z13.web.core.windows.net
How To Calculate Dilution Concentrations Dilution Factor Using Concentration The purpose of a dilution is to decrease the concentration of the solution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Learn how to use the dilution equation. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilution Factor Using Concentration The purpose of a dilution is to decrease the concentration of the solution. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn the formula, components, and applications of dilution factor in scientific research. Learn how to calculate the concentration and volume of a diluted solution using the. Dilution Factor Using Concentration.
From www.researchgate.net
Why do antigen concentrations measured in ELISA change depending on Dilution Factor Using Concentration Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn the formula, components, and applications of dilution factor in scientific research. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Learn how to use. Dilution Factor Using Concentration.
From www.pdfprof.com
how to calculate dilution factor Dilution Factor Using Concentration Since the number of moles of solute must remain constant, the only way to achieve the. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Factor Using Concentration Find out how to avoid common mistakes, understand. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Since the number of moles of solute must remain constant, the only way. Dilution Factor Using Concentration.
From www.researchgate.net
The sample, dilution factor, and BOD values and the calculated Dilution Factor Using Concentration Find out how to avoid common mistakes, understand. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. The dilution factor (or dilution ratio) is the. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Using Concentration Since the number of moles of solute must remain constant, the only way to achieve the. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after. Dilution Factor Using Concentration.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Factor Using Concentration The purpose of a dilution is to decrease the concentration of the solution. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Learn the formula, components, and applications of dilution factor in scientific research. Find out how to avoid common mistakes, understand. The dilution factor (or dilution ratio) is the notation used to. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Using Concentration Learn the formula, components, and applications of dilution factor in scientific research. The purpose of a dilution is to decrease the concentration of the solution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Find out how to avoid common mistakes, understand. Learn how to. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution Factor Using Concentration The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Since the number of moles of solute must remain constant, the only way to achieve the. Find out how to. Dilution Factor Using Concentration.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Factor Using Concentration Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn how to calculate the dilution factor, a ratio or exponent that describes the. Dilution Factor Using Concentration.
From www.youtube.com
Calculating the concentration of a solution using a dilution factor Dilution Factor Using Concentration Find out how to avoid common mistakes, understand. Since the number of moles of solute must remain constant, the only way to achieve the. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn how to calculate the concentration and volume of a diluted solution using the dilution. Dilution Factor Using Concentration.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Factor Using Concentration Since the number of moles of solute must remain constant, the only way to achieve the. Learn the formula, components, and applications of dilution factor in scientific research. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to calculate the dilution factor, a. Dilution Factor Using Concentration.
From www.vrogue.co
What Is Serial Dilution Method And How To Calculate S vrogue.co Dilution Factor Using Concentration Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. The purpose of a dilution is to decrease the concentration of the solution. Find out how to avoid common mistakes, understand. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of. Dilution Factor Using Concentration.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA Dilution Factor Using Concentration Find out how to avoid common mistakes, understand. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn the formula, components, and applications. Dilution Factor Using Concentration.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Factor Using Concentration Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. The purpose of a dilution is to decrease the concentration of the solution. Find out how to avoid common mistakes, understand. Since the number of moles of solute must remain constant, the only way to. Dilution Factor Using Concentration.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Factor Using Concentration Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Find out how to avoid common mistakes, understand. The purpose of a dilution is to decrease the concentration of the solution. Since the number of moles of solute must remain constant, the only way to achieve the. Learn how to calculate the dilution factor,. Dilution Factor Using Concentration.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution Factor Using Concentration Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Since the number of moles of solute must remain constant, the only way to achieve the. Learn the formula, components, and applications of dilution factor in scientific research. The purpose of a dilution is to decrease the concentration of. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Dilution, Concentration and Alligation PowerPoint Presentation Dilution Factor Using Concentration The purpose of a dilution is to decrease the concentration of the solution. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn the. Dilution Factor Using Concentration.
From www.researchgate.net
Correlation equation of concentration with dilution factor using Dilution Factor Using Concentration Find out how to avoid common mistakes, understand. Learn the formula, components, and applications of dilution factor in scientific research. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or. Dilution Factor Using Concentration.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Factor Using Concentration Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to use the dilution equation m1v1 = m2v2 to calculate the. Dilution Factor Using Concentration.
From www.slideshare.net
Molarity and dilution Dilution Factor Using Concentration Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Learn the formula, components, and applications of dilution factor in scientific research.. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Concentration PowerPoint Presentation, free download ID2186169 Dilution Factor Using Concentration Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Learn the formula, components, and applications of dilution factor in scientific research. Since the number of moles of solute must remain constant, the only way to achieve the. The dilution factor (or dilution ratio) is the notation. Dilution Factor Using Concentration.
From www.slideshare.net
Molarity and dilution Dilution Factor Using Concentration Learn how to calculate the dilution factor, a ratio or exponent that describes the reduction in concentration of a solute in a solution. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Since the number of moles of solute must remain constant, the only. Dilution Factor Using Concentration.
From materialmagicpayne.z21.web.core.windows.net
How To Calculate Dilution Concentrations Dilution Factor Using Concentration Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Find out how to avoid common mistakes, understand. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn the formula, components, and applications. Dilution Factor Using Concentration.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Factor Using Concentration The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The purpose of a dilution is to decrease the concentration of the solution. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. Dilution Factor Using Concentration.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Factor Using Concentration Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to calculate the dilution factor of a stock solution when making a diluted solution with molar or percent concentration. Since the number of moles of solute must remain constant, the only way to. Dilution Factor Using Concentration.
From materialmagicpayne.z21.web.core.windows.net
How To Calculate Dilution Concentrations Dilution Factor Using Concentration The purpose of a dilution is to decrease the concentration of the solution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Find out how to avoid common mistakes,. Dilution Factor Using Concentration.