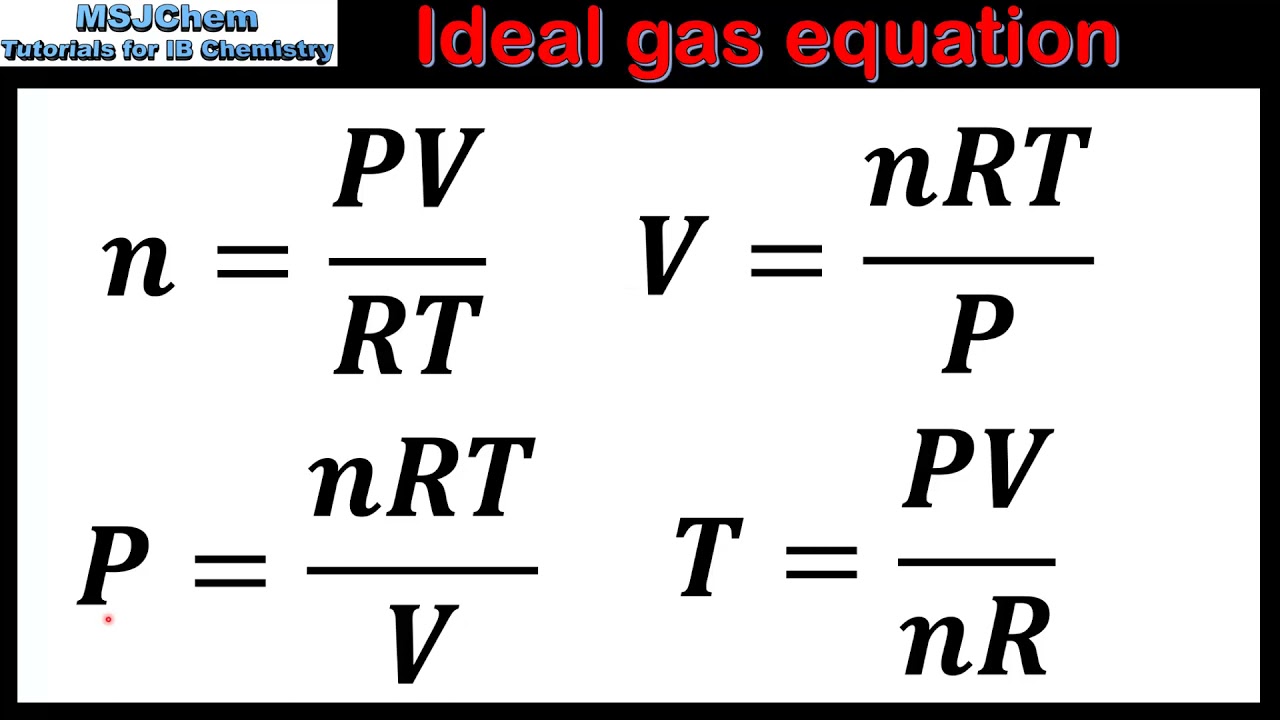
Correction Factor A In Ideal Gas Equation . In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. There are two corrective factors in van der waals equation. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. To account for deviation from the ideal situation an other. The first, , alters the pressure in the ideal gas equation. The ideal gas law is accurate only at relatively low pressures and high temperatures. Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. Greater the value of ′a′, more.
from www.youtube.com
The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. Greater the value of ′a′, more. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. To account for deviation from the ideal situation an other. In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. The first, , alters the pressure in the ideal gas equation. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. There are two corrective factors in van der waals equation. The ideal gas law is accurate only at relatively low pressures and high temperatures.
1.3 Ideal gas equation YouTube
Correction Factor A In Ideal Gas Equation Greater the value of ′a′, more. The first, , alters the pressure in the ideal gas equation. The ideal gas law is accurate only at relatively low pressures and high temperatures. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. Greater the value of ′a′, more. There are two corrective factors in van der waals equation. In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. To account for deviation from the ideal situation an other. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Correction Factor A In Ideal Gas Equation There are two corrective factors in van der waals equation. Greater the value of ′a′, more. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is. Correction Factor A In Ideal Gas Equation.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Correction Factor A In Ideal Gas Equation Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. It is defined as \[pv = zrt\] w here. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
The Tds equations for an ideal gas YouTube Correction Factor A In Ideal Gas Equation The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. It is defined as \[pv = zrt\] w here \[z\] \[v\]. Greater the value of ′a′, more. The ideal gas law is accurate only at relatively low pressures and high temperatures. To account for deviation from. Correction Factor A In Ideal Gas Equation.
From www.grc.nasa.gov
Equation of State Correction Factor A In Ideal Gas Equation There are two corrective factors in van der waals equation. The ideal gas law is accurate only at relatively low pressures and high temperatures. To account for deviation from the ideal situation an other. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The first,. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
Thermodynamics 37 Ideal Gas Equation with compressibility factor Correction Factor A In Ideal Gas Equation The ideal gas law is accurate only at relatively low pressures and high temperatures. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. The first, , alters the pressure in the ideal gas equation. Therefore a full correction to the. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
The correction factor \'a\' to the ideal gas equation corresponds to Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. Greater the value of ′a′, more. To account for deviation from the ideal situation an other. The ideal gas law is accurate only at relatively low pressures and high temperatures. The correction. Correction Factor A In Ideal Gas Equation.
From www.toppr.com
Correct gas equation is Chemistry Questions Correction Factor A In Ideal Gas Equation There are two corrective factors in van der waals equation. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. The first, , alters the pressure in the ideal gas equation. The compressibility factor is a dimensionless correction factor to account. Correction Factor A In Ideal Gas Equation.
From slideplayer.com
States of Matter Lesson ppt download Correction Factor A In Ideal Gas Equation It is defined as \[pv = zrt\] w here \[z\] \[v\]. The ideal gas law is accurate only at relatively low pressures and high temperatures. To account for deviation from the ideal situation an other. The first, , alters the pressure in the ideal gas equation. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction. Correction Factor A In Ideal Gas Equation.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry Correction Factor A In Ideal Gas Equation It is defined as \[pv = zrt\] w here \[z\] \[v\]. Greater the value of ′a′, more. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. The first, , alters the pressure in the ideal gas equation. In the van der waals equation, the pressure correction. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
NonIdeal Gases and the Van der Waals Equation YouTube Correction Factor A In Ideal Gas Equation Greater the value of ′a′, more. It is defined as \[pv = zrt\] w here \[z\] \[v\]. To account for deviation from the ideal situation an other. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
1.3 Ideal gas equation YouTube Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. The first, , alters the. Correction Factor A In Ideal Gas Equation.
From ar.inspiredpencil.com
Non Ideal Gas Equations Correction Factor A In Ideal Gas Equation Greater the value of ′a′, more. There are two corrective factors in van der waals equation. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. The first, , alters the pressure in the ideal gas equation. To account for deviation from the ideal situation an other.. Correction Factor A In Ideal Gas Equation.
From www.numerade.com
SOLVED In the idealgas equation, could an equivalent Celsius Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. The ideal gas law is. Correction Factor A In Ideal Gas Equation.
From www.toppr.com
At low pressure, Van der Waal's equation is reduced to [P + aV^2] V Correction Factor A In Ideal Gas Equation To account for deviation from the ideal situation an other. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. The first, , alters the pressure in the ideal gas equation. The ideal gas law is accurate only at relatively low pressures and high temperatures. It. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
Mass and volume flow rates Ideal Gas Example YouTube Correction Factor A In Ideal Gas Equation Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. To account for deviation from the ideal situation an other. The ideal gas law is accurate only at relatively low pressures and high temperatures. It is defined as \[pv = zrt\] w here. Correction Factor A In Ideal Gas Equation.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Correction Factor A In Ideal Gas Equation To account for deviation from the ideal situation an other. The first, , alters the pressure in the ideal gas equation. There are two corrective factors in van der waals equation. In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. The. Correction Factor A In Ideal Gas Equation.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. Therefore a full correction to the ideal gas. Correction Factor A In Ideal Gas Equation.
From www.dailymotion.com
Ideal Gas Equation Crash Course Chemistry 12 Derivation of pv=nrt Correction Factor A In Ideal Gas Equation The ideal gas law is accurate only at relatively low pressures and high temperatures. To account for deviation from the ideal situation an other. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. There are two corrective factors in van der waals equation. The correction factor. Correction Factor A In Ideal Gas Equation.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Correction Factor A In Ideal Gas Equation There are two corrective factors in van der waals equation. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. To account for deviation from the ideal situation an other. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas. Correction Factor A In Ideal Gas Equation.
From mmerevise.co.uk
The Ideal Gas Equation MME Correction Factor A In Ideal Gas Equation The first, , alters the pressure in the ideal gas equation. To account for deviation from the ideal situation an other. There are two corrective factors in van der waals equation. The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. Greater the value of ′a′,. Correction Factor A In Ideal Gas Equation.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. The ideal gas law is accurate only at relatively low pressures and high temperatures. To account for deviation from the ideal situation an other. The ideal gas law is derived from empirical. Correction Factor A In Ideal Gas Equation.
From www.studocu.com
Ideal Gas Equations and Laws Studocu Correction Factor A In Ideal Gas Equation Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. There are two corrective factors in van der waals equation.. Correction Factor A In Ideal Gas Equation.
From en.ppt-online.org
The ideal gas equation online presentation Correction Factor A In Ideal Gas Equation The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. To account for deviation from the ideal situation an other. There are two corrective factors in van der waals equation. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The compressibility factor is a dimensionless correction factor to account for. Correction Factor A In Ideal Gas Equation.
From www.slideserve.com
PPT Thermodynamic Properties PowerPoint Presentation, free download Correction Factor A In Ideal Gas Equation Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. There are two corrective factors. Correction Factor A In Ideal Gas Equation.
From www.coursehero.com
[Solved] Using the ideal gas equation calculate the volume that 15 g of Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The ideal gas law is accurate only at relatively low pressures and high temperatures. The correction factor ′a′ to the ideal. Correction Factor A In Ideal Gas Equation.
From ar.inspiredpencil.com
Non Ideal Gas Equations Correction Factor A In Ideal Gas Equation The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. Greater the value of ′a′, more. In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. The first, , alters the pressure in the. Correction Factor A In Ideal Gas Equation.
From askfilo.com
StatementI The correction factor ' a ' in the real gas equation corresp.. Correction Factor A In Ideal Gas Equation The first, , alters the pressure in the ideal gas equation. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. Therefore a full correction to the ideal gas law, which we call the van der waals equation, keeps the nrt side intact but changes the left side. The ideal gas law. Correction Factor A In Ideal Gas Equation.
From pressbooks.bccampus.ca
3.2 Real gas and compressibility factor Introduction to Engineering Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. It is defined as \[pv = zrt\] w here \[z\] \[v\]. There are two corrective factors in van der waals equation. Therefore a full correction to the ideal gas law, which we. Correction Factor A In Ideal Gas Equation.
From byjus.com
Different forms of ideal gas equation Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. It is defined as \[pv = zrt\] w here \[z\] \[v\]. There are two corrective factors in van der waals equation. The ideal gas law is derived from empirical relationships among the. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
Chemistry A Level calculations Applying the ideal gas equation YouTube Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. The first, , alters the pressure in the ideal. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
The correction factor ' \( a \) ' in the ideal gas equation corresp Correction Factor A In Ideal Gas Equation There are two corrective factors in van der waals equation. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The ideal gas law is accurate only at relatively low pressures and high temperatures. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. The first,. Correction Factor A In Ideal Gas Equation.
From www.myxxgirl.com
Ideal Gas Law Constant Unit My XXX Hot Girl Correction Factor A In Ideal Gas Equation The first, , alters the pressure in the ideal gas equation. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. Greater the value of ′a′, more. It is. Correction Factor A In Ideal Gas Equation.
From www.studypool.com
SOLUTION Applications of ideal gas equation Studypool Correction Factor A In Ideal Gas Equation In the van der waals equation, the pressure correction factor ‘a’ accounts for the attractive forces between gas molecules, which is not considered in the ideal gas law. The ideal gas law is accurate only at relatively low pressures and high temperatures. Greater the value of ′a′, more. The correction factor ′a′ to the ideal gas equation corresponds to forces. Correction Factor A In Ideal Gas Equation.
From slideplayer.com
Quinnipiac University ppt download Correction Factor A In Ideal Gas Equation The first, , alters the pressure in the ideal gas equation. Greater the value of ′a′, more. It is defined as \[pv = zrt\] w here \[z\] \[v\]. The correction factor ′a′ to the ideal gas equation corresponds to forces of attraction between the gas molecules. There are two corrective factors in van der waals equation. Therefore a full correction. Correction Factor A In Ideal Gas Equation.
From www.youtube.com
Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes! YouTube Correction Factor A In Ideal Gas Equation The ideal gas law is derived from empirical relationships among the pressure, the volume, the temperature, and the number of moles of a gas;. Greater the value of ′a′, more. The compressibility factor is a dimensionless correction factor to account for the deviation of the real gas behaviour from the “ideal” gas model. The ideal gas law is accurate only. Correction Factor A In Ideal Gas Equation.