Copper Acetate Dissolve In Water . If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. Refer to the chart below to find reference values. It can dissolve in polar solvents due to the presence of acetate ions. water temperature can have a significant effect on the solubility of compounds. [2] copper (ii) acetate has a color that is. cupric acetate exhibits moderate solubility in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. what do we mean by solubility of a salt? Soluble in h 2 o (6.79 g/100 ml, 25 °c);
from chart-studio.plotly.com
If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. cupric acetate exhibits moderate solubility in water. Refer to the chart below to find reference values. [2] copper (ii) acetate has a color that is. water temperature can have a significant effect on the solubility of compounds. Soluble in h 2 o (6.79 g/100 ml, 25 °c); It can dissolve in polar solvents due to the presence of acetate ions. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. what do we mean by solubility of a salt?
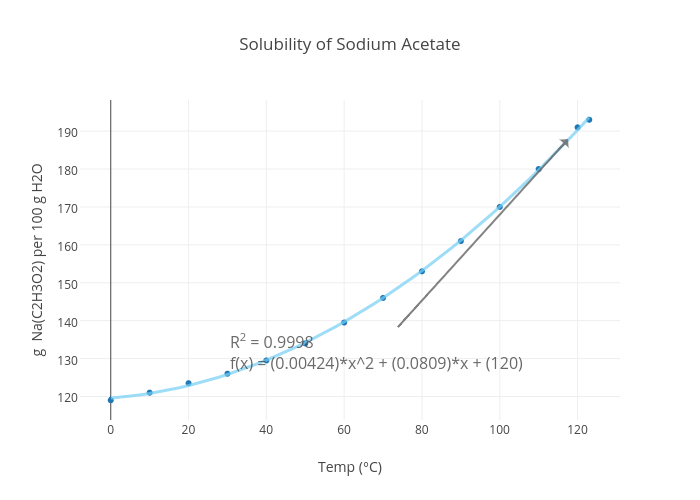
Solubility of Sodium Acetate scatter chart made by Mrericsully plotly
Copper Acetate Dissolve In Water Soluble in h 2 o (6.79 g/100 ml, 25 °c); cupric acetate exhibits moderate solubility in water. water temperature can have a significant effect on the solubility of compounds. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. Refer to the chart below to find reference values. It can dissolve in polar solvents due to the presence of acetate ions. Soluble in h 2 o (6.79 g/100 ml, 25 °c); [2] copper (ii) acetate has a color that is. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. what do we mean by solubility of a salt?
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Copper Acetate Dissolve In Water what do we mean by solubility of a salt? It can dissolve in polar solvents due to the presence of acetate ions. water temperature can have a significant effect on the solubility of compounds. cupric acetate exhibits moderate solubility in water. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly. Copper Acetate Dissolve In Water.
From chem.libretexts.org
13.3 Factors Affecting Solubility Chemistry LibreTexts Copper Acetate Dissolve In Water Refer to the chart below to find reference values. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. [2] copper (ii) acetate has a color that is. cupric. Copper Acetate Dissolve In Water.
From dxogtzdjb.blob.core.windows.net
Copper Hydroxide Is Soluble In Water at Linwood blog Copper Acetate Dissolve In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. [2] copper (ii) acetate has a color that is. It can dissolve in polar solvents due to the presence of. Copper Acetate Dissolve In Water.
From woelen.homescience.net
Science made alive Chemistry/Experiments Copper Acetate Dissolve In Water cupric acetate exhibits moderate solubility in water. [2] copper (ii) acetate has a color that is. what do we mean by solubility of a salt? Soluble in h 2 o (6.79 g/100 ml, 25 °c); It can dissolve in polar solvents due to the presence of acetate ions. Refer to the chart below to find reference values. If. Copper Acetate Dissolve In Water.
From dmishin.github.io
Copper Acetate Copper Acetate Dissolve In Water Refer to the chart below to find reference values. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. cupric acetate exhibits moderate solubility in water. Soluble in h 2 o (6.79 g/100 ml, 25 °c); If i say one mole of sodium sulfate dissolves in water. Copper Acetate Dissolve In Water.
From www.aliexpress.com
Water solubility Copper Peptide GHKCU Glycyllhistidylllysine 1gram Copper Acetate Dissolve In Water [2] copper (ii) acetate has a color that is. Refer to the chart below to find reference values. cupric acetate exhibits moderate solubility in water. what do we mean by solubility of a salt? If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. water temperature can have a. Copper Acetate Dissolve In Water.
From www.researchgate.net
Temperature dependence of the solubility of copper sulfate in water Copper Acetate Dissolve In Water [2] copper (ii) acetate has a color that is. cupric acetate exhibits moderate solubility in water. water temperature can have a significant effect on the solubility of compounds. Soluble in h 2 o (6.79 g/100 ml, 25 °c); It can dissolve in polar solvents due to the presence of acetate ions. what do we mean by solubility. Copper Acetate Dissolve In Water.
From www.nagwa.com
Question Video Describing the Type of Solution Produced by Dissolving Copper Acetate Dissolve In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. [2] copper (ii) acetate has a color that is. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. what do we mean by solubility of a salt? . Copper Acetate Dissolve In Water.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Copper Acetate Dissolve In Water cupric acetate exhibits moderate solubility in water. Refer to the chart below to find reference values. what do we mean by solubility of a salt? It can dissolve in polar solvents due to the presence of acetate ions. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because. Copper Acetate Dissolve In Water.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Copper Acetate Dissolve In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. [2] copper (ii) acetate has a color that is. Soluble in h 2 o (6.79 g/100 ml, 25 °c); It can dissolve in polar solvents due to the presence of acetate ions. If i say one mole of. Copper Acetate Dissolve In Water.
From dxonxarcb.blob.core.windows.net
What Type Of Substances Will Dissolve In Water To Form Aqueous Copper Acetate Dissolve In Water water temperature can have a significant effect on the solubility of compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. [2] copper (ii) acetate has a color that is. Refer to the chart below to find reference values. what do we mean by solubility. Copper Acetate Dissolve In Water.
From www.researchgate.net
6. Solubility versus pH curves for the thermodynamically stable Copper Acetate Dissolve In Water what do we mean by solubility of a salt? Refer to the chart below to find reference values. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. cupric acetate exhibits moderate solubility in water. It can dissolve in polar solvents due to the presence of. Copper Acetate Dissolve In Water.
From dmishin.github.io
Calcium Copper Acetate Copper Acetate Dissolve In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. Soluble in h 2 o (6.79 g/100 ml, 25 °c); It can dissolve in polar solvents due to the presence. Copper Acetate Dissolve In Water.
From chart-studio.plotly.com
Solubility of Sodium Acetate scatter chart made by Mrericsully plotly Copper Acetate Dissolve In Water cupric acetate exhibits moderate solubility in water. Soluble in h 2 o (6.79 g/100 ml, 25 °c); water temperature can have a significant effect on the solubility of compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. [2] copper (ii) acetate has a color. Copper Acetate Dissolve In Water.
From www.reddit.com
Some copper acetate solution I’m evaporating r/crystalgrowing Copper Acetate Dissolve In Water It can dissolve in polar solvents due to the presence of acetate ions. [2] copper (ii) acetate has a color that is. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. cupric acetate exhibits moderate solubility in water. water temperature can have a significant effect. Copper Acetate Dissolve In Water.
From www.researchgate.net
Solubility of lead (II) acetate and formate in water at different Copper Acetate Dissolve In Water what do we mean by solubility of a salt? Soluble in h 2 o (6.79 g/100 ml, 25 °c); cupric acetate exhibits moderate solubility in water. water temperature can have a significant effect on the solubility of compounds. [2] copper (ii) acetate has a color that is. when ionic compounds dissolve in water, the ions in. Copper Acetate Dissolve In Water.
From www.researchgate.net
Electronic spectra of the reaction between copper(II) acetate, sodium Copper Acetate Dissolve In Water what do we mean by solubility of a salt? water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. Soluble in h 2 o (6.79 g/100 ml, 25 °c);. Copper Acetate Dissolve In Water.
From dmishin.github.io
Copper Acetate Copper Acetate Dissolve In Water Soluble in h 2 o (6.79 g/100 ml, 25 °c); water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values. [2] copper (ii) acetate has a color that is. cupric acetate exhibits moderate solubility in water. If i say one mole of sodium sulfate dissolves in water. Copper Acetate Dissolve In Water.
From dmishin.github.io
Calcium Copper Acetate Copper Acetate Dissolve In Water [2] copper (ii) acetate has a color that is. Soluble in h 2 o (6.79 g/100 ml, 25 °c); It can dissolve in polar solvents due to the presence of acetate ions. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. cupric acetate exhibits moderate solubility in water. what. Copper Acetate Dissolve In Water.
From dmishin.github.io
Calcium Copper Acetate Copper Acetate Dissolve In Water [2] copper (ii) acetate has a color that is. It can dissolve in polar solvents due to the presence of acetate ions. water temperature can have a significant effect on the solubility of compounds. when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. cupric acetate. Copper Acetate Dissolve In Water.
From www.flinnsci.ca
Solubility Rules Chart, Notebook Size, Pad of 30 Flinn Scientific Copper Acetate Dissolve In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. cupric acetate exhibits moderate solubility in water. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. what do we mean by solubility of a salt? [2] copper. Copper Acetate Dissolve In Water.
From www.youtube.com
Synthesis of Copper Acetate(II) Tutorial and Explanation YouTube Copper Acetate Dissolve In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. Refer to the chart below to find reference values. cupric acetate exhibits moderate solubility in water. It can dissolve in polar solvents due to the presence of acetate ions. water temperature can have a significant effect. Copper Acetate Dissolve In Water.
From www.youtube.com
How to Write the Formula for Copper (II) acetate YouTube Copper Acetate Dissolve In Water what do we mean by solubility of a salt? water temperature can have a significant effect on the solubility of compounds. It can dissolve in polar solvents due to the presence of acetate ions. Soluble in h 2 o (6.79 g/100 ml, 25 °c); Refer to the chart below to find reference values. cupric acetate exhibits moderate. Copper Acetate Dissolve In Water.
From www.semanticscholar.org
[PDF] Solubility, liquidliquid equilibrium and critical states for the Copper Acetate Dissolve In Water cupric acetate exhibits moderate solubility in water. [2] copper (ii) acetate has a color that is. water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values. It can dissolve in polar solvents due to the presence of acetate ions. what do we mean by solubility of. Copper Acetate Dissolve In Water.
From www.researchgate.net
Solubility of ethyl acetate in water and saturation concentration of Copper Acetate Dissolve In Water cupric acetate exhibits moderate solubility in water. [2] copper (ii) acetate has a color that is. what do we mean by solubility of a salt? water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values. Soluble in h 2 o (6.79 g/100 ml, 25 °c); If. Copper Acetate Dissolve In Water.
From www.mdpi.com
IJMS Free FullText Solubility of Amino Acids in the Eutectic Copper Acetate Dissolve In Water Refer to the chart below to find reference values. Soluble in h 2 o (6.79 g/100 ml, 25 °c); If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. water temperature can have a significant effect on the solubility of compounds. what do we mean by solubility of a salt?. Copper Acetate Dissolve In Water.
From dmishin.github.io
Copper Acetate Copper Acetate Dissolve In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. [2] copper (ii) acetate has a color that is. water temperature can have a significant effect on the solubility of compounds. Refer to the chart below to find reference values. It can dissolve in polar solvents due to the presence of. Copper Acetate Dissolve In Water.
From cymitquimica.com
Copper(II) Acetate Monohydrate CymitQuimica Copper Acetate Dissolve In Water [2] copper (ii) acetate has a color that is. what do we mean by solubility of a salt? when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. . Copper Acetate Dissolve In Water.
From www.youtube.com
How to make a Blue Solution of Copper(II) acetate from Pennies, Vinegar Copper Acetate Dissolve In Water [2] copper (ii) acetate has a color that is. cupric acetate exhibits moderate solubility in water. water temperature can have a significant effect on the solubility of compounds. Soluble in h 2 o (6.79 g/100 ml, 25 °c); If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. Refer to. Copper Acetate Dissolve In Water.
From www.researchgate.net
(PDF) SOLUTE STRUCTURE OF COPPER(II)ACETATE SOLUTIONS IN LIQUID AND Copper Acetate Dissolve In Water water temperature can have a significant effect on the solubility of compounds. It can dissolve in polar solvents due to the presence of acetate ions. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. what do we mean by solubility of a salt? [2] copper (ii) acetate has a. Copper Acetate Dissolve In Water.
From mungfali.com
Solubility Chemistry Diagram Copper Acetate Dissolve In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. Refer to the chart below to find reference values. Soluble in h 2 o (6.79 g/100 ml, 25 °c); when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. . Copper Acetate Dissolve In Water.
From dmishin.github.io
Copper Acetate Copper Acetate Dissolve In Water when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules. water temperature can have a significant effect on the solubility of compounds. It can dissolve in polar solvents due to the presence of acetate ions. cupric acetate exhibits moderate solubility in water. Soluble in h 2. Copper Acetate Dissolve In Water.
From sciencenotes.org
Grow BlueGreen Copper Acetate Crystals Copper Acetate Dissolve In Water what do we mean by solubility of a salt? It can dissolve in polar solvents due to the presence of acetate ions. Refer to the chart below to find reference values. Soluble in h 2 o (6.79 g/100 ml, 25 °c); when ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. Copper Acetate Dissolve In Water.
From www.researchgate.net
(PDF) Dependence of the Solubility of Basic Copper Acetate on the Copper Acetate Dissolve In Water Soluble in h 2 o (6.79 g/100 ml, 25 °c); If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. Refer to the chart below to find reference values. water temperature can have a significant effect on the solubility of compounds. what do we mean by solubility of a salt?. Copper Acetate Dissolve In Water.
From www.911metallurgist.com
Acid Solubility of Copper Mineral Species Copper Acetate Dissolve In Water [2] copper (ii) acetate has a color that is. Soluble in h 2 o (6.79 g/100 ml, 25 °c); It can dissolve in polar solvents due to the presence of acetate ions. If i say one mole of sodium sulfate dissolves in water i am really meaning for every mole. cupric acetate exhibits moderate solubility in water. water. Copper Acetate Dissolve In Water.