Endpoint Vs Equivalence Point Titration . Point) versus endpoint is that the equivalence point (eq. endpoint and equivalence point are two terms commonly used in titration experiments. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. The endpoint refers to the. the major difference between equivalence (eq.
from chem.libretexts.org
endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. the major difference between equivalence (eq. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. Point) versus endpoint is that the equivalence point (eq. The endpoint refers to the.
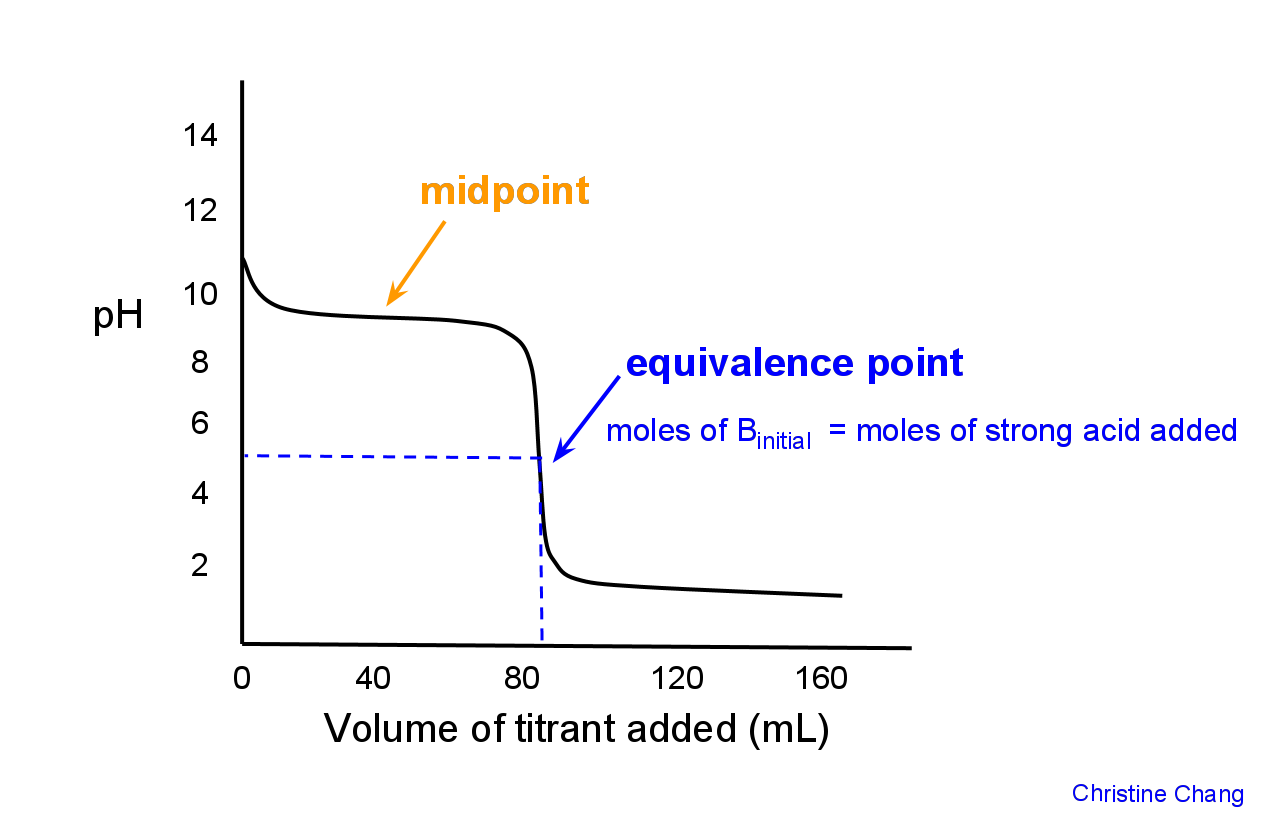
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Endpoint Vs Equivalence Point Titration the major difference between equivalence (eq. the major difference between equivalence (eq. The endpoint refers to the. Point) versus endpoint is that the equivalence point (eq. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species.
From www.numerade.com
Question 4 (30 marks) a) Compare between equivalence point and endpoint Endpoint Vs Equivalence Point Titration endpoint and equivalence point are two terms commonly used in titration experiments. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other. Endpoint Vs Equivalence Point Titration.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Endpoint Vs Equivalence Point Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. Point) versus endpoint is that the equivalence point (eq. endpoint and equivalence point. Endpoint Vs Equivalence Point Titration.
From ar.inspiredpencil.com
Equivalence Point And Endpoint Endpoint Vs Equivalence Point Titration endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The endpoint refers to the. . Endpoint Vs Equivalence Point Titration.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition Endpoint Vs Equivalence Point Titration the major difference between equivalence (eq. The endpoint refers to the. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. Endpoint Vs Equivalence Point Titration.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Endpoint Vs Equivalence Point Titration learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. The endpoint refers to the. the major difference between equivalence (eq. Point) versus endpoint is that the equivalence point (eq. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Endpoint Vs Equivalence Point Titration.
From askanydifference.com
Endpoint vs Equivalence Point Difference and Comparison Endpoint Vs Equivalence Point Titration The endpoint refers to the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint and equivalence point are two terms commonly used in titration experiments. learn the difference between endpoint and equivalence point in titration, a method to determine acids,. Endpoint Vs Equivalence Point Titration.
From thecontentauthority.com
Equivalence Point vs Endpoint Meaning And Differences Endpoint Vs Equivalence Point Titration the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. Point) versus endpoint is that the equivalence point (eq. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence. Endpoint Vs Equivalence Point Titration.
From www.numerade.com
SOLVED The different between endpoint vs equivalence point which one Endpoint Vs Equivalence Point Titration endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. the major difference between equivalence. Endpoint Vs Equivalence Point Titration.
From mavink.com
Titration Endpoint Vs Equivalence Point Endpoint Vs Equivalence Point Titration endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. Point) versus endpoint is that the equivalence point (eq. endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the. the major difference between. Endpoint Vs Equivalence Point Titration.
From www.numerade.com
Explain the difference between equivalence point and endpoint Consider Endpoint Vs Equivalence Point Titration Point) versus endpoint is that the equivalence point (eq. The endpoint refers to the. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. the major difference between equivalence (eq. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Endpoint Vs Equivalence Point Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Endpoint Vs Equivalence Point Titration endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. Point) versus endpoint is that the equivalence point (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. the major. Endpoint Vs Equivalence Point Titration.
From www.sciencemotive.com
Differentiate Between Endpoint and Equivalence Point ScienceMotive Endpoint Vs Equivalence Point Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. Point) versus endpoint is that the equivalence point (eq. the major difference between. Endpoint Vs Equivalence Point Titration.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Endpoint Vs Equivalence Point Titration Point) versus endpoint is that the equivalence point (eq. The endpoint refers to the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint and equivalence point are two terms commonly used in titration experiments. the major difference between equivalence (eq.. Endpoint Vs Equivalence Point Titration.
From www.difference.wiki
Equivalence Point vs. Endpoint What’s the Difference? Endpoint Vs Equivalence Point Titration The endpoint refers to the. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species.. Endpoint Vs Equivalence Point Titration.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Endpoint Vs Equivalence Point Titration Point) versus endpoint is that the equivalence point (eq. endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint is the stage in titration that is indicated by a. Endpoint Vs Equivalence Point Titration.
From www.numerade.com
SOLVED What is the difference between the endpoint and the equivalence Endpoint Vs Equivalence Point Titration The endpoint refers to the. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. Point) versus endpoint is that the equivalence point (eq. the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration,. Endpoint Vs Equivalence Point Titration.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Endpoint Vs Equivalence Point Titration learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Endpoint Vs Equivalence Point Titration.
From grammarbeast.com
Endpoint vs Equivalence Point Choosing the Right One To Use Endpoint Vs Equivalence Point Titration The endpoint refers to the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint and equivalence point are two terms commonly used in titration experiments. the major difference between equivalence (eq. endpoint is the stage in titration that is. Endpoint Vs Equivalence Point Titration.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Endpoint Vs Equivalence Point Titration Point) versus endpoint is that the equivalence point (eq. endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint is the stage in titration that is indicated by a. Endpoint Vs Equivalence Point Titration.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Endpoint Vs Equivalence Point Titration endpoint and equivalence point are two terms commonly used in titration experiments. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other. Endpoint Vs Equivalence Point Titration.
From www.differencebetween.com
Difference Between Endpoint and Stoichiometric Point Compare the Endpoint Vs Equivalence Point Titration learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. Point) versus endpoint is that the equivalence point (eq. endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added. Endpoint Vs Equivalence Point Titration.
From edurev.in
End Point, Equivalence Point and Indicators Physical Chemistry PDF Endpoint Vs Equivalence Point Titration the major difference between equivalence (eq. The endpoint refers to the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and. Endpoint Vs Equivalence Point Titration.
From www.numerade.com
SOLVEDWhat is the difference between the endpoint and the equivalence Endpoint Vs Equivalence Point Titration endpoint and equivalence point are two terms commonly used in titration experiments. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The endpoint refers to the. endpoint is the stage in titration that is indicated by a color change as a. Endpoint Vs Equivalence Point Titration.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Endpoint Vs Equivalence Point Titration Point) versus endpoint is that the equivalence point (eq. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. endpoint and equivalence point are two terms commonly used in titration experiments. the major difference between equivalence (eq. learn the. Endpoint Vs Equivalence Point Titration.
From www.youtube.com
What is the difference between the endpoint and equivalence point in a Endpoint Vs Equivalence Point Titration endpoint and equivalence point are two terms commonly used in titration experiments. Point) versus endpoint is that the equivalence point (eq. the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. a titration is a volumetric technique in which a solution. Endpoint Vs Equivalence Point Titration.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Endpoint Vs Equivalence Point Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint and equivalence point are two terms commonly used in titration experiments. the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration, a method to. Endpoint Vs Equivalence Point Titration.
From mavink.com
Titration Endpoint Vs Equivalence Point Endpoint Vs Equivalence Point Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. Point) versus endpoint is that the equivalence point (eq. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point. Endpoint Vs Equivalence Point Titration.
From www.vrogue.co
Endpoint Vs Equivalence Point Ppt Powerpoint Presenta vrogue.co Endpoint Vs Equivalence Point Titration Point) versus endpoint is that the equivalence point (eq. The endpoint refers to the. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. endpoint and equivalence point are two terms commonly used in titration experiments. endpoint is the stage in titration. Endpoint Vs Equivalence Point Titration.
From romeo-blogmeza.blogspot.com
Explain Equivalence Point and End Point Endpoint Vs Equivalence Point Titration a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. endpoint is the stage in titration. Endpoint Vs Equivalence Point Titration.
From www.numerade.com
SOLVED What is the difference between the endpoint and the equivalence Endpoint Vs Equivalence Point Titration the major difference between equivalence (eq. endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Endpoint Vs Equivalence Point Titration.
From animalia-life.club
Endpoint Titration Endpoint Vs Equivalence Point Titration endpoint is the stage in titration that is indicated by a color change as a sign that titration is complete and the equivalence point has been achieved. Point) versus endpoint is that the equivalence point (eq. the major difference between equivalence (eq. The endpoint refers to the. a titration is a volumetric technique in which a solution. Endpoint Vs Equivalence Point Titration.
From www.vrogue.co
Endpoint Vs Equivalence Point Understanding The Diffe vrogue.co Endpoint Vs Equivalence Point Titration Point) versus endpoint is that the equivalence point (eq. endpoint and equivalence point are two terms commonly used in titration experiments. the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. a titration is a volumetric technique in which a solution. Endpoint Vs Equivalence Point Titration.
From mavink.com
Titration Endpoint Vs Equivalence Point Endpoint Vs Equivalence Point Titration the major difference between equivalence (eq. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The endpoint refers to the. endpoint and equivalence point are two terms commonly used in titration experiments. learn the difference between endpoint and equivalence point. Endpoint Vs Equivalence Point Titration.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Endpoint Vs Equivalence Point Titration The endpoint refers to the. endpoint and equivalence point are two terms commonly used in titration experiments. Point) versus endpoint is that the equivalence point (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. a titration is a volumetric technique in which a solution of one. Endpoint Vs Equivalence Point Titration.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Endpoint Vs Equivalence Point Titration the major difference between equivalence (eq. learn the difference between endpoint and equivalence point in titration, a method to determine acids, bases and other species. The endpoint refers to the. Point) versus endpoint is that the equivalence point (eq. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Endpoint Vs Equivalence Point Titration.