Magnesium And Zinc Nitrate Reaction Observations . Changes in color, temperature, gas. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. No reaction occurs between magnesium. • iron filings or small nails are of low hazard. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. chemical reactions are ubiquitous and used to understand and explain how our world works. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it.
from www.chegg.com
magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. chemical reactions are ubiquitous and used to understand and explain how our world works. No reaction occurs between magnesium. • iron filings or small nails are of low hazard. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. Changes in color, temperature, gas.
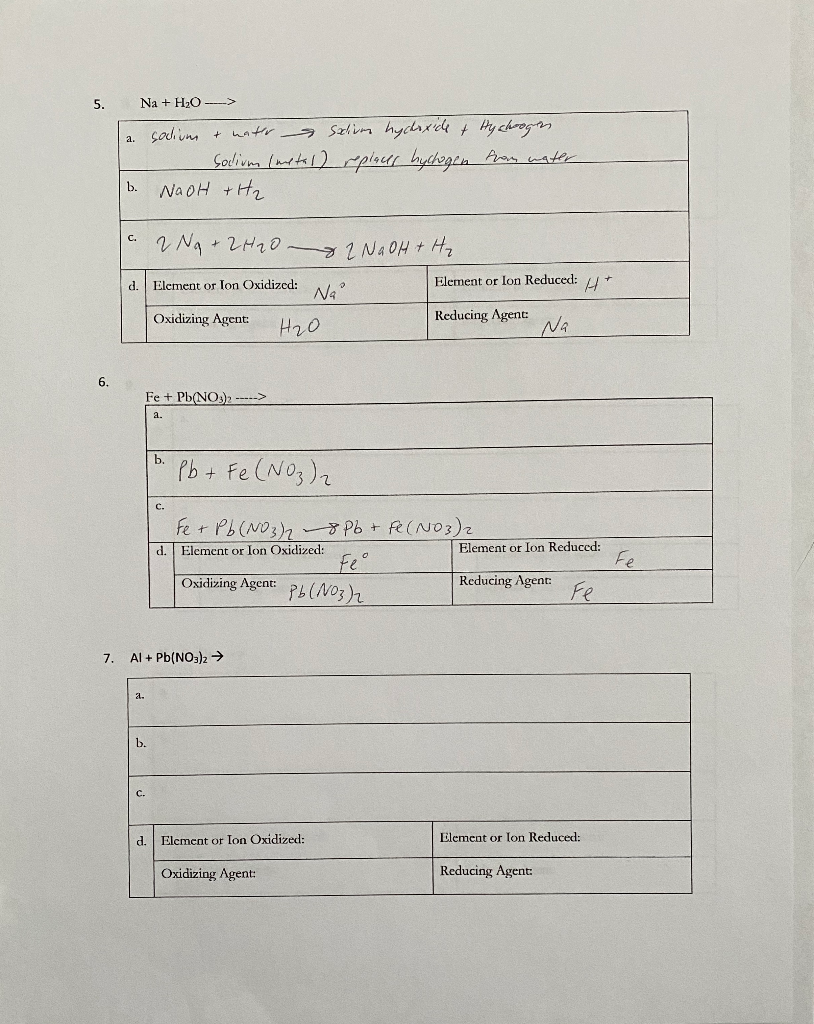
Solved EXAMPLES 1. Mg + Zn(NO3)2 > a. Magnesium + zinc
Magnesium And Zinc Nitrate Reaction Observations magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. No reaction occurs between magnesium. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. chemical reactions are ubiquitous and used to understand and explain how our world works. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. Changes in color, temperature, gas. • iron filings or small nails are of low hazard. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.21 Practical Investigate Reactions Between Magnesium And Zinc Nitrate Reaction Observations Changes in color, temperature, gas. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. chemical reactions are ubiquitous and used to understand and explain how our world works. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. No reaction occurs between magnesium. — since \(\ce{mg}\). Magnesium And Zinc Nitrate Reaction Observations.
From www.chegg.com
Solved Use the interactive to observe the reactions between Magnesium And Zinc Nitrate Reaction Observations — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. Changes in color, temperature, gas. chemical reactions are ubiquitous and used to understand and explain how our world works. 9 rows —. Magnesium And Zinc Nitrate Reaction Observations.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. chemical reactions are ubiquitous and used to understand and explain how our world works. • iron filings or small nails are of low hazard. metals can be arranged in order of reactivity by observing their reactions with water, acid and. Magnesium And Zinc Nitrate Reaction Observations.
From www.youtube.com
Reaction of Copper with Zinc and Magnesium Nitrate YouTube Magnesium And Zinc Nitrate Reaction Observations • iron filings or small nails are of low hazard. chemical reactions are ubiquitous and used to understand and explain how our world works. Changes in color, temperature, gas. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a. Magnesium And Zinc Nitrate Reaction Observations.
From www.chegg.com
Solved EXAMPLES 1. Mg + Zn(NO3)2 > a. Magnesium + zinc Magnesium And Zinc Nitrate Reaction Observations Changes in color, temperature, gas. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. chemical reactions are ubiquitous and used to understand and explain how our world works. No reaction occurs between magnesium. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. • iron filings or. Magnesium And Zinc Nitrate Reaction Observations.
From www.youtube.com
WHEN ZINC NITRATE REACTS WITH MAGNESIUM RIBBON YouTube Magnesium And Zinc Nitrate Reaction Observations — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. magnesium. Magnesium And Zinc Nitrate Reaction Observations.
From www.meritnation.com
Pl solve this 3 Complete the following word equations and write Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. Changes in color, temperature, gas. • iron filings or small nails are of low hazard. metals can be arranged in order of reactivity by observing their reactions with. Magnesium And Zinc Nitrate Reaction Observations.
From www.chegg.com
REPORT SHEET LAB Chemical Reactions and Equations 8 Magnesium And Zinc Nitrate Reaction Observations metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. • iron filings or small nails are of low hazard. chemical reactions are ubiquitous and used to understand and explain how our world works. Changes in color, temperature, gas. 9 rows — we can examine the reactivity. Magnesium And Zinc Nitrate Reaction Observations.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. Changes in color, temperature, gas. chemical reactions are ubiquitous and used to understand and explain how our world works. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it.. Magnesium And Zinc Nitrate Reaction Observations.
From www.chegg.com
Solved CHEMISTRY. REACTIONS IN SOLUTION Lab Data х Reaction Magnesium And Zinc Nitrate Reaction Observations magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. — since \(\ce{mg}\). Magnesium And Zinc Nitrate Reaction Observations.
From www.bartleby.com
Answered Data and Observations Change or… bartleby Magnesium And Zinc Nitrate Reaction Observations 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. • iron filings or small nails are of low hazard. metals can be arranged in order of reactivity by observing their reactions. Magnesium And Zinc Nitrate Reaction Observations.
From slideplayer.com
Patterns of Reactivity ppt download Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. No reaction occurs between magnesium. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. • iron filings or small nails are of low. Magnesium And Zinc Nitrate Reaction Observations.
From exobevaom.blob.core.windows.net
Products Of Magnesium And Zinc Nitrate at Roberto Olson blog Magnesium And Zinc Nitrate Reaction Observations magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. No reaction occurs between magnesium. • iron filings or small nails are of low hazard. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. chemical reactions are ubiquitous and used to understand and. Magnesium And Zinc Nitrate Reaction Observations.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. No reaction occurs between magnesium. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. chemical reactions are ubiquitous and used to understand and explain how our world works. — since \(\ce{mg}\) is more active than \(\ce{al}\),. Magnesium And Zinc Nitrate Reaction Observations.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. No reaction occurs between magnesium. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. •. Magnesium And Zinc Nitrate Reaction Observations.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Magnesium And Zinc Nitrate Reaction Observations metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. • iron filings or small nails are of low hazard. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single. Magnesium And Zinc Nitrate Reaction Observations.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + Zn(NO3)2 = Mg(NO3)2 + Zn Magnesium And Zinc Nitrate Reaction Observations magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. • iron filings or small nails are of low hazard. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. Changes in color, temperature, gas. 9 rows — we can examine the reactivity of metals by observing their. Magnesium And Zinc Nitrate Reaction Observations.
From www.coursehero.com
[Solved] Magnesium nitrate reacts with barium in a single displacement Magnesium And Zinc Nitrate Reaction Observations 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. Changes in color, temperature, gas. • iron filings or small nails are of low hazard. chemical reactions are ubiquitous and used to. Magnesium And Zinc Nitrate Reaction Observations.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID639260 Magnesium And Zinc Nitrate Reaction Observations magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. • iron filings or small nails are of low hazard. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. . Magnesium And Zinc Nitrate Reaction Observations.
From schematicmemberfdiche.z22.web.core.windows.net
Magnesium And Lead Reaction Magnesium And Zinc Nitrate Reaction Observations chemical reactions are ubiquitous and used to understand and explain how our world works. Changes in color, temperature, gas. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. No reaction occurs between magnesium. • iron filings or. Magnesium And Zinc Nitrate Reaction Observations.
From www.nagwa.com
Question Video Identifying the Correct Observation When Magnesium Is Magnesium And Zinc Nitrate Reaction Observations No reaction occurs between magnesium. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. chemical reactions are ubiquitous and used to understand and explain how our world works. • iron filings or small nails are of low hazard. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. Changes in. Magnesium And Zinc Nitrate Reaction Observations.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. • iron filings or small nails are of low hazard. magnesium and zinc react with the iron(iii) nitrate, the solution. Magnesium And Zinc Nitrate Reaction Observations.
From www.chemedx.org
Demonstration Reaction of Magnesium and Silver Nitrate Chemical Magnesium And Zinc Nitrate Reaction Observations 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. Changes in color, temperature, gas. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. metals can be arranged in order of reactivity by observing their reactions with water,. Magnesium And Zinc Nitrate Reaction Observations.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Magnesium And Zinc Nitrate Reaction Observations — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. chemical reactions are ubiquitous and used to understand and explain how our world works. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. revision notes on 2.2.3 thermal decomposition of. Magnesium And Zinc Nitrate Reaction Observations.
From www.youtube.com
Ionic Reactions Magnesium Nitrate + Sodium Sulfate YouTube Magnesium And Zinc Nitrate Reaction Observations • iron filings or small nails are of low hazard. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. No reaction occurs between magnesium. revision notes on 2.2.3 thermal decomposition of nitrates. Magnesium And Zinc Nitrate Reaction Observations.
From www.numerade.com
SOLVED When zinc metal is placed into copper(II) nitrate solution Magnesium And Zinc Nitrate Reaction Observations • iron filings or small nails are of low hazard. chemical reactions are ubiquitous and used to understand and explain how our world works. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. Changes in color, temperature, gas. 9 rows — we can examine the reactivity of metals by observing their reactions. Magnesium And Zinc Nitrate Reaction Observations.
From www.chegg.com
Solved EXAMPLES 1. Mg + Zn(NO3)2 > a. Magnesium + zinc Magnesium And Zinc Nitrate Reaction Observations No reaction occurs between magnesium. Changes in color, temperature, gas. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. • iron filings or small nails are of low hazard. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. revision notes. Magnesium And Zinc Nitrate Reaction Observations.
From www.youtube.com
Reaction of Zinc in Magnesium and Copper Nitrate YouTube Magnesium And Zinc Nitrate Reaction Observations • iron filings or small nails are of low hazard. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions. Magnesium And Zinc Nitrate Reaction Observations.
From www.transtutors.com
(Solved) EXAMPLES 1. Mg + Zn(NO3)2 > a. Magnesium + zinc nitrate Magnesium And Zinc Nitrate Reaction Observations — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. No reaction occurs between magnesium. . Magnesium And Zinc Nitrate Reaction Observations.
From autoctrls.com
The Fascinating Insight into the Magnesium Zinc Phase Diagram Magnesium And Zinc Nitrate Reaction Observations • iron filings or small nails are of low hazard. chemical reactions are ubiquitous and used to understand and explain how our world works. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. No reaction occurs between magnesium. — since \(\ce{mg}\) is more active than. Magnesium And Zinc Nitrate Reaction Observations.
From www.youtube.com
Magnesium Sulfate + Zinc YouTube Magnesium And Zinc Nitrate Reaction Observations chemical reactions are ubiquitous and used to understand and explain how our world works. No reaction occurs between magnesium. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,.. Magnesium And Zinc Nitrate Reaction Observations.
From www.alamy.com
Labelled diagram of magnesium reacting with steam. Vector diagram for Magnesium And Zinc Nitrate Reaction Observations • iron filings or small nails are of low hazard. chemical reactions are ubiquitous and used to understand and explain how our world works. Changes in color, temperature, gas. metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other. — since \(\ce{mg}\) is more active than \(\ce{al}\),. Magnesium And Zinc Nitrate Reaction Observations.
From chemistryexperimentphotogallery.blogspot.com
Chemistry Experiment Photo Gallery October 2010 Magnesium And Zinc Nitrate Reaction Observations chemical reactions are ubiquitous and used to understand and explain how our world works. — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. 9 rows — we can examine the reactivity of metals by. Magnesium And Zinc Nitrate Reaction Observations.
From www.numerade.com
SOLVED 'er on test tube hydroxide and magnesium nitrate were mixed Magnesium And Zinc Nitrate Reaction Observations revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. No reaction occurs between magnesium. 9 rows — we can examine the reactivity of metals by observing their reactions with oxygen, water, steam and whether it. magnesium and zinc react with the iron(iii) nitrate, the solution gradually darkens. • iron. Magnesium And Zinc Nitrate Reaction Observations.
From www.slideserve.com
PPT Single Displacement Reactions PowerPoint Presentation, free Magnesium And Zinc Nitrate Reaction Observations — since \(\ce{mg}\) is more active than \(\ce{al}\), a single displacement reaction will occur. Changes in color, temperature, gas. chemical reactions are ubiquitous and used to understand and explain how our world works. revision notes on 2.2.3 thermal decomposition of nitrates & carbonates for the cie a level chemistry syllabus,. • iron filings or small nails are. Magnesium And Zinc Nitrate Reaction Observations.