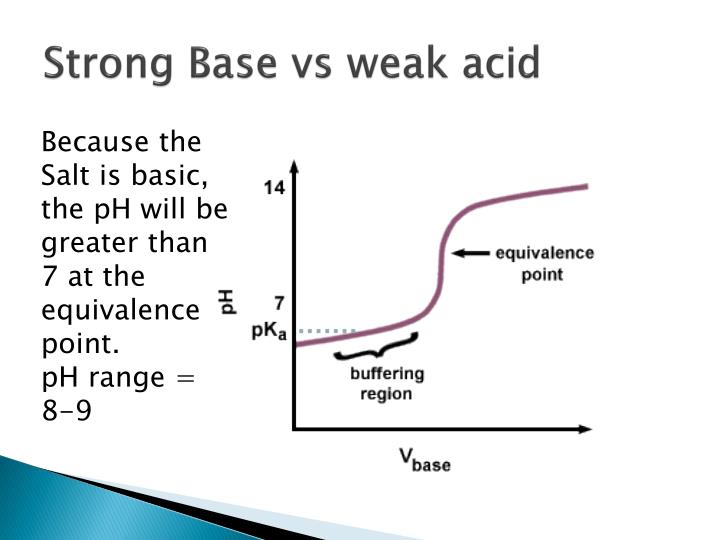
Weak Acid Strong Base Titration Equivalence Point Ph . The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. the molarity of the acid is given, so the number of moles titrated can be calculated: It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. 0.050 l × 6 mol/l = 0.3 moles. A conjugate acid will be.
from www.slideserve.com
It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. 0.050 l × 6 mol/l = 0.3 moles. A conjugate acid will be. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. the molarity of the acid is given, so the number of moles titrated can be calculated: for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an.
PPT Acid Base Titrations PowerPoint Presentation ID2145680
Weak Acid Strong Base Titration Equivalence Point Ph the molarity of the acid is given, so the number of moles titrated can be calculated: you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. the molarity of the acid is given, so the number of moles titrated can be calculated: in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. A conjugate acid will be. 0.050 l × 6 mol/l = 0.3 moles. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Weak Acid Strong Base Titration Equivalence Point Ph A conjugate acid will be. 0.050 l × 6 mol/l = 0.3 moles. the molarity of the acid is given, so the number of moles titrated can be calculated: in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. The reaction of the. Weak Acid Strong Base Titration Equivalence Point Ph.
From capechemistry.blogspot.com
CAPE CHEMISTRY Weak Base Strong Acid Titration Curves Weak Acid Strong Base Titration Equivalence Point Ph you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a weak base with a strong acid, the. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid Weak Acid Strong Base Titration Equivalence Point Ph It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. in a titration of a strong acid with a strong. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Weak Acid Strong Base Titration Equivalence Point Ph 0.050 l × 6 mol/l = 0.3 moles. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. the molarity of the acid is given, so the. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid Weak Acid Strong Base Titration Equivalence Point Ph the molarity of the acid is given, so the number of moles titrated can be calculated: for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. A conjugate acid will be. in a titration of a strong acid with a strong base, or a strong base. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.slideshare.net
pH Understanding titration curve Weak Acid Strong Base Titration Equivalence Point Ph It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. A conjugate acid will be. in a titration of a strong acid with a strong base, or a strong. Weak Acid Strong Base Titration Equivalence Point Ph.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Weak Acid Strong Base Titration Equivalence Point Ph the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. A conjugate acid will be. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The reaction of the. Weak Acid Strong Base Titration Equivalence Point Ph.
From srkzzxycbyqbm.blogspot.com
How To Find Ph At Equivalence Point Weak Acid Strong Base However Weak Acid Strong Base Titration Equivalence Point Ph 0.050 l × 6 mol/l = 0.3 moles. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A conjugate acid will be.. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Weak Acid Strong Base Titration Equivalence Point Ph the molarity of the acid is given, so the number of moles titrated can be calculated: the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a weak base with a strong acid, the ph curve is initially basic. Weak Acid Strong Base Titration Equivalence Point Ph.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Weak Acid Strong Base Titration Equivalence Point Ph you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. 0.050 l × 6 mol/l = 0.3 moles. in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. for the titration. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.chm.uri.edu
CHM 112 Lecture 19 Weak Acid Strong Base Titration Equivalence Point Ph 0.050 l × 6 mol/l = 0.3 moles. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. the molarity of the acid is. Weak Acid Strong Base Titration Equivalence Point Ph.
From saylordotorg.github.io
AcidBase Titrations Weak Acid Strong Base Titration Equivalence Point Ph the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. you can use this same approach to calculate the titration curve for. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.youtube.com
17.3 Calculating the pH at the Equivalence Point of a Weak Acid Strong Weak Acid Strong Base Titration Equivalence Point Ph A conjugate acid will be. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. 0.050 l × 6 mol/l = 0.3 moles. the molarity. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at Weak Acid Strong Base Titration Equivalence Point Ph It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A conjugate acid will be. you can use this same approach. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube Weak Acid Strong Base Titration Equivalence Point Ph the molarity of the acid is given, so the number of moles titrated can be calculated: It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. in a titration of a strong acid with a strong base, or a strong base with a strong acid,. Weak Acid Strong Base Titration Equivalence Point Ph.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Weak Acid Strong Base Titration Equivalence Point Ph the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. the molarity of the acid is given, so the number of moles titrated can be calculated: the titration of a weak acid with a strong base involves the direct transfer of protons. Weak Acid Strong Base Titration Equivalence Point Ph.
From boisestate.pressbooks.pub
14.7 AcidBase Titrations General Chemistry 1 & 2 Weak Acid Strong Base Titration Equivalence Point Ph for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. the titration curve for the titration of 25.00 ml of 0.100. Weak Acid Strong Base Titration Equivalence Point Ph.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Weak Acid Strong Base Titration Equivalence Point Ph in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. A conjugate acid will be. the molarity of the acid. Weak Acid Strong Base Titration Equivalence Point Ph.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Weak Acid Strong Base Titration Equivalence Point Ph the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. you can use this same approach to calculate the titration curve. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation ID2145680 Weak Acid Strong Base Titration Equivalence Point Ph the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. 0.050 l × 6 mol/l = 0.3 moles. It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. in a titration. Weak Acid Strong Base Titration Equivalence Point Ph.
From saylordotorg.github.io
AcidBase Titrations Weak Acid Strong Base Titration Equivalence Point Ph the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. 0.050 l × 6 mol/l = 0.3 moles. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. A conjugate acid will. Weak Acid Strong Base Titration Equivalence Point Ph.
From gregorycbroman.blob.core.windows.net
Titration Definition Wiki at gregorycbroman blog Weak Acid Strong Base Titration Equivalence Point Ph the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. It is 8.75 , and this is because of the accumulated acetate. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.vrogue.co
Strong Acid And Strong Base Titration Curve vrogue.co Weak Acid Strong Base Titration Equivalence Point Ph the molarity of the acid is given, so the number of moles titrated can be calculated: in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. you can use this same approach to calculate the titration curve for the titration of a. Weak Acid Strong Base Titration Equivalence Point Ph.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Weak Acid Strong Base Titration Equivalence Point Ph It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. A conjugate acid will be. in a titration of a strong acid with a. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Weak Acid Strong Base Titration Equivalence Point Ph the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A conjugate acid will be. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. 0.050 l × 6 mol/l = 0.3 moles. the titration curve for. Weak Acid Strong Base Titration Equivalence Point Ph.
From chemistry.stackexchange.com
Shape of WeakStrong AcidBase Titration Chemistry Stack Exchange Weak Acid Strong Base Titration Equivalence Point Ph A conjugate acid will be. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially, changes. you can. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Weak Acid Strong Base Titration Equivalence Point Ph A conjugate acid will be. 0.050 l × 6 mol/l = 0.3 moles. It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. the molarity of the acid is given, so the number of moles titrated can be calculated: the titration of a weak acid. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.reddit.com
Is (half equivalence point) when a conjugate base and an acid are equal Weak Acid Strong Base Titration Equivalence Point Ph A conjugate acid will be. the molarity of the acid is given, so the number of moles titrated can be calculated: for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. 0.050 l × 6 mol/l = 0.3 moles. It is 8.75 , and this is because. Weak Acid Strong Base Titration Equivalence Point Ph.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Weak Acid Strong Base Titration Equivalence Point Ph for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. 0.050 l × 6 mol/l = 0.3 moles. the molarity of the acid is given, so the number of moles titrated can be calculated: It is 8.75 , and this is because of the accumulated acetate ion. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Weak Acid Strong Base Titration Equivalence Point Ph 0.050 l × 6 mol/l = 0.3 moles. The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. in a titration of a strong acid with a. Weak Acid Strong Base Titration Equivalence Point Ph.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Weak Acid Strong Base Titration Equivalence Point Ph The reaction of the weak acid, acetic acid, with a strong base, naoh, can be seen below. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. A conjugate acid will be. in a titration of a strong acid with a strong base, or a strong base. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Weak Acid Strong Base Titration Equivalence Point Ph 0.050 l × 6 mol/l = 0.3 moles. It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. you can use. Weak Acid Strong Base Titration Equivalence Point Ph.
From vdocuments.mx
Titration of a weak acid with a strong base pH calculations 1. Before Weak Acid Strong Base Titration Equivalence Point Ph for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. A conjugate acid will be. 0.050 l × 6 mol/l = 0.3 moles. in a titration of a strong acid with a strong base, or a strong base with a strong acid, the ph changes slowly initially,. Weak Acid Strong Base Titration Equivalence Point Ph.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Weak Acid Strong Base Titration Equivalence Point Ph you can use this same approach to calculate the titration curve for the titration of a weak base with a strong. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the molarity of the acid is given, so the number of moles. Weak Acid Strong Base Titration Equivalence Point Ph.
From socratic.org
The "pH" at onehalf the equivalence point in an acidbase titration Weak Acid Strong Base Titration Equivalence Point Ph It is 8.75 , and this is because of the accumulated acetate ion which is formed as the acetic acid reacts with naoh. for the titration of a weak base with a strong acid, the ph curve is initially basic and has an acidic. you can use this same approach to calculate the titration curve for the titration. Weak Acid Strong Base Titration Equivalence Point Ph.