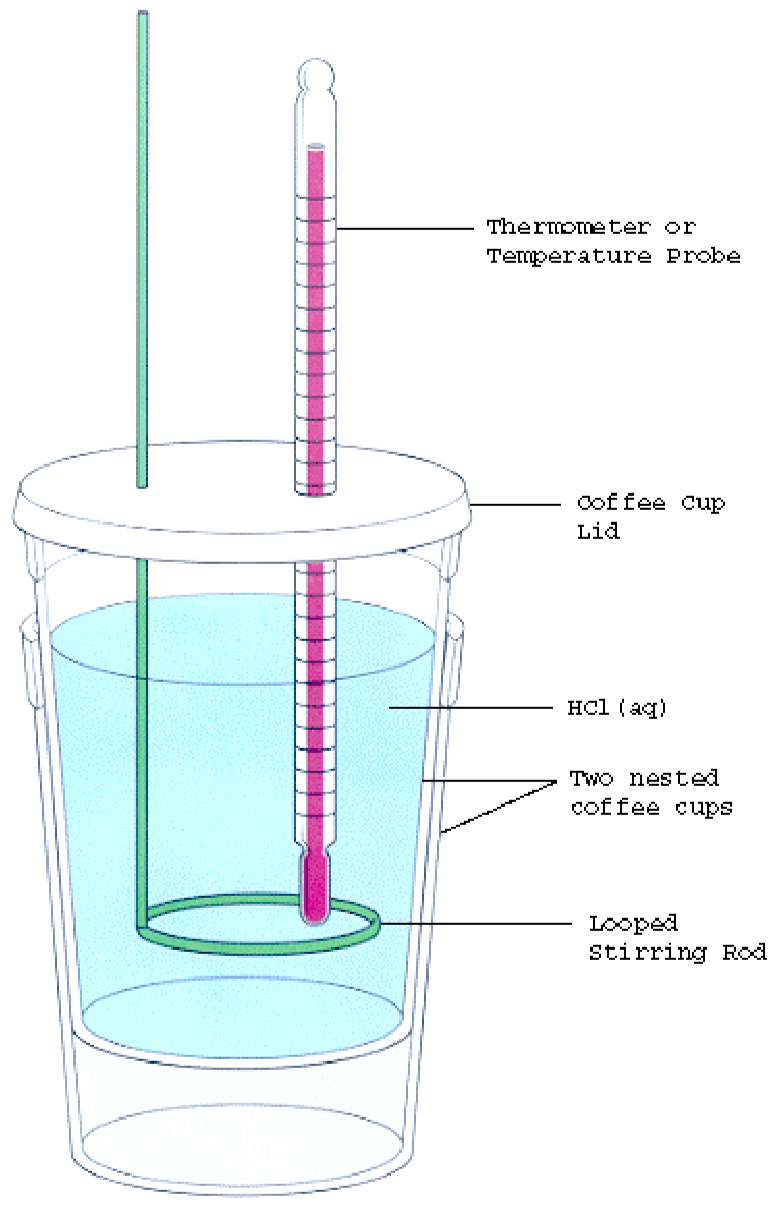
Heat Effects And Calorimetry Lab . Heat always flows from high. Heat is a form of energy that is transferred between objects with different temperatures. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is used to measure. 98 experiment 14 heat effects and calorimetry. In this experiment we measure the masses of water and metal and their initial and final temperatures. Lab session 9, experiment 8: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determination of the heat of solution. Determination of the specific heat of a metal.
from chem.libretexts.org
Determination of the specific heat of a metal. Lab session 9, experiment 8: 98 experiment 14 heat effects and calorimetry. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. In this experiment we measure the masses of water and metal and their initial and final temperatures. Heat is a form of energy that is transferred between objects with different temperatures. Calorimetry is used to measure. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determination of the heat of solution. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Heat Effects And Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. 98 experiment 14 heat effects and calorimetry. Heat always flows from high. Heat is a form of energy that is transferred between objects with different temperatures. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Determination of the heat of solution. Determination of the specific heat of a metal. Lab session 9, experiment 8: In this experiment we measure the masses of water and metal and their initial and final temperatures.
From www.chegg.com
Solved Heat Effects And Calorimetry Lab Report with data Heat Effects And Calorimetry Lab Heat always flows from high. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. 98 experiment 14 heat effects and calorimetry. Determination of the specific heat of a metal. Determination of the heat of solution. Heat is a. Heat Effects And Calorimetry Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Heat Effects And Calorimetry Lab 98 experiment 14 heat effects and calorimetry. In this experiment we measure the masses of water and metal and their initial and final temperatures. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Calorimetry is used to measure.. Heat Effects And Calorimetry Lab.
From www.pdfprof.com
calorimétrie pdf Heat Effects And Calorimetry Lab The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. In this experiment we measure the masses of water and metal and their initial and final temperatures. One technique we can use to measure the amount of heat involved. Heat Effects And Calorimetry Lab.
From www.studypool.com
SOLUTION Online experiment heat effects and calorimetry Studypool Heat Effects And Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. In this experiment we measure the masses of water and metal and their initial and final temperatures. Determination of the specific heat of a metal. The objectives of the experiment were achieved as the molar masses and specific heat values. Heat Effects And Calorimetry Lab.
From studylib.net
Calorimetry Lab Specific Heat Capacity Heat Effects And Calorimetry Lab Heat is a form of energy that is transferred between objects with different temperatures. Determination of the specific heat of a metal. 98 experiment 14 heat effects and calorimetry. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: Determination of the heat of solution.. Heat Effects And Calorimetry Lab.
From www.chegg.com
Experiment 14 Data and Calculations Heat Effects and Heat Effects And Calorimetry Lab Determination of the heat of solution. Lab session 9, experiment 8: 98 experiment 14 heat effects and calorimetry. Calorimetry is used to measure. Determination of the specific heat of a metal. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the. Heat Effects And Calorimetry Lab.
From library.achievingthedream.org
Calorimetry Introductory Chemistry Lecture & Lab Heat Effects And Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Heat always flows from high. Determination of. Heat Effects And Calorimetry Lab.
From cityraven.com
👍 Heat effects and calorimetry lab answers. Heat Effects And Heat Effects And Calorimetry Lab Determination of the specific heat of a metal. 98 experiment 14 heat effects and calorimetry. Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Heat is a form of energy that is transferred between objects with different temperatures. Determination of the heat of solution.. Heat Effects And Calorimetry Lab.
From users.highland.edu
Calorimetry Heat Effects And Calorimetry Lab The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Determination of the heat of solution. In this experiment we measure the masses of water and metal and their initial and final temperatures. 98 experiment 14 heat effects and. Heat Effects And Calorimetry Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Heat Effects And Calorimetry Lab Determination of the heat of solution. In this experiment we measure the masses of water and metal and their initial and final temperatures. Determination of the specific heat of a metal. Heat is a form of energy that is transferred between objects with different temperatures. Calorimetry is used to measure. Lab session 9, experiment 8: Heat always flows from high.. Heat Effects And Calorimetry Lab.
From www.studocu.com
Lab Manual 5 Heat Effects and Calorimetry Lab Report Experiment 14 Heat Effects And Calorimetry Lab 98 experiment 14 heat effects and calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is used to measure. Heat always flows from high. Determination. Heat Effects And Calorimetry Lab.
From www.coursehero.com
[Solved] Experiment 7 Heat Effects and Calorimetry PreLaboratory Heat Effects And Calorimetry Lab 98 experiment 14 heat effects and calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat is a form of energy that is transferred between objects with different temperatures. The objectives of the experiment were achieved as the molar masses and specific heat values of the. Heat Effects And Calorimetry Lab.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Heat Effects And Calorimetry Lab 98 experiment 14 heat effects and calorimetry. Heat is a form of energy that is transferred between objects with different temperatures. Determination of the specific heat of a metal. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. Heat always flows from. Heat Effects And Calorimetry Lab.
From humberto.perka.org
Experiment 14 Heat Effects And Calorimetry Advance Study Assignment Heat Effects And Calorimetry Lab Determination of the heat of solution. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Heat is a form of energy that is transferred between objects with different temperatures. Calorimetry is used to measure. Specific heat is an. Heat Effects And Calorimetry Lab.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Heat Effects And Calorimetry Lab The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Heat always flows from high. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determination of the heat. Heat Effects And Calorimetry Lab.
From www.scribd.com
2 Heat Effects and Calorimetry PDF Calorimetry Enthalpy Heat Effects And Calorimetry Lab Determination of the specific heat of a metal. Determination of the heat of solution. Lab session 9, experiment 8: 98 experiment 14 heat effects and calorimetry. Calorimetry is used to measure. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment we measure the masses. Heat Effects And Calorimetry Lab.
From www.chegg.com
Solved CHM 2045L Heat Effects and Calorimetry PostLab Heat Effects And Calorimetry Lab Heat always flows from high. 98 experiment 14 heat effects and calorimetry. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Heat Effects And Calorimetry Lab.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download Heat Effects And Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determination of the specific heat of a metal. Calorimetry is used to measure. In this experiment we measure the masses of water and metal and their initial and final temperatures. Heat always flows from high. One technique we can use. Heat Effects And Calorimetry Lab.
From www.chegg.com
106 Experiment 14 Heat Effects and Calorimetry d. Heat Effects And Calorimetry Lab In this experiment we measure the masses of water and metal and their initial and final temperatures. Determination of the specific heat of a metal. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat always flows from high. Determination of the heat of solution. Lab session. Heat Effects And Calorimetry Lab.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Heat Effects And Calorimetry Lab Heat always flows from high. Heat is a form of energy that is transferred between objects with different temperatures. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. In this experiment we measure the masses of water and metal and their initial and final temperatures. Calorimetry is used to. Heat Effects And Calorimetry Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Heat Effects And Calorimetry Lab In this experiment we measure the masses of water and metal and their initial and final temperatures. Heat always flows from high. Determination of the specific heat of a metal. Calorimetry is used to measure. 98 experiment 14 heat effects and calorimetry. Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or. Heat Effects And Calorimetry Lab.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Heat Effects And Calorimetry Lab The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. 98 experiment 14 heat effects and calorimetry. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determination of. Heat Effects And Calorimetry Lab.
From www.chegg.com
Solved Heat Effects And Calorimetry Advance Study Can Som... Heat Effects And Calorimetry Lab Calorimetry is used to measure. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. In this experiment we measure the masses of water and metal and their initial and final temperatures. One technique we can use to measure. Heat Effects And Calorimetry Lab.
From www.chegg.com
Mass of calorimeter Mass of calorimeter and water Heat Effects And Calorimetry Lab In this experiment we measure the masses of water and metal and their initial and final temperatures. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. 98 experiment 14 heat effects and calorimetry. Heat is a form of energy that is transferred between objects with different temperatures.. Heat Effects And Calorimetry Lab.
From www.studypool.com
SOLUTION Online experiment heat effects and calorimetry Studypool Heat Effects And Calorimetry Lab In this experiment we measure the masses of water and metal and their initial and final temperatures. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Heat is a form of energy that is transferred between objects with. Heat Effects And Calorimetry Lab.
From www.bartleby.com
Answered Experiment 14 Data and Calculations… bartleby Heat Effects And Calorimetry Lab In this experiment we measure the masses of water and metal and their initial and final temperatures. Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determination of the specific heat of a metal. One technique we can use to measure the amount of. Heat Effects And Calorimetry Lab.
From www.chegg.com
Experiment 14 Data and Calculations Heat Effects and Heat Effects And Calorimetry Lab Determination of the heat of solution. 98 experiment 14 heat effects and calorimetry. Heat always flows from high. Heat is a form of energy that is transferred between objects with different temperatures. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values,. Heat Effects And Calorimetry Lab.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Heat Effects And Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat is a form of energy that is transferred between objects with different temperatures. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The objectives of the. Heat Effects And Calorimetry Lab.
From www.studocu.com
Heat Effects and Calorimetry Lab Report Name Akzhan Yestoreyeva Date Heat Effects And Calorimetry Lab Heat always flows from high. 98 experiment 14 heat effects and calorimetry. Determination of the heat of solution. Lab session 9, experiment 8: Determination of the specific heat of a metal. Calorimetry is used to measure. Heat is a form of energy that is transferred between objects with different temperatures. In this experiment we measure the masses of water and. Heat Effects And Calorimetry Lab.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Heat Effects And Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determination of the heat of solution. In this experiment we measure the masses of water and metal and their initial and final temperatures. Heat always flows from high. Heat is a form of energy that is transferred between objects with. Heat Effects And Calorimetry Lab.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Heat Effects And Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The objectives of the experiment were achieved as the molar masses and specific heat values of the four unknown metals were calculated by using the heat effect values, the heat of. Heat is a form of energy that. Heat Effects And Calorimetry Lab.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Heat Effects And Calorimetry Lab Determination of the heat of solution. Calorimetry is used to measure. In this experiment we measure the masses of water and metal and their initial and final temperatures. 98 experiment 14 heat effects and calorimetry. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determination of the specific heat. Heat Effects And Calorimetry Lab.
From www.studypool.com
SOLUTION Online experiment heat effects and calorimetry Studypool Heat Effects And Calorimetry Lab Heat always flows from high. Determination of the specific heat of a metal. Calorimetry is used to measure. Lab session 9, experiment 8: Determination of the heat of solution. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The objectives of the experiment were achieved as the. Heat Effects And Calorimetry Lab.
From www.coursehero.com
[Solved] Heat Effects And Calorimetry Lab Report with data Table 1 Heat Effects And Calorimetry Lab In this experiment we measure the masses of water and metal and their initial and final temperatures. Calorimetry is used to measure. Determination of the heat of solution. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. 98 experiment 14 heat effects and calorimetry. Lab session 9,. Heat Effects And Calorimetry Lab.
From www.chegg.com
Solved Name Section Experiment 14 Data And Calculations Heat Effects And Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is used to measure. Heat is a form of energy that is transferred between objects with different temperatures. Determination of the heat of solution. 98 experiment 14 heat effects and calorimetry. In this experiment we measure the masses of. Heat Effects And Calorimetry Lab.