Acid-Base Indicators Lab Report . This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. To understand relationship between ph and h+ ion concentration. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. Acids and bases can be transformed when their. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. To learn to use a laboratory ph meter. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. To understand ph differences of acids and bases. In titrations indicators help in the. In this article, the author has explained acid base titration, its working principle types and process of acid base titration.
from studylib.net
A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. To learn to use a laboratory ph meter. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. To understand ph differences of acids and bases. In titrations indicators help in the. Acids and bases can be transformed when their. To understand relationship between ph and h+ ion concentration.
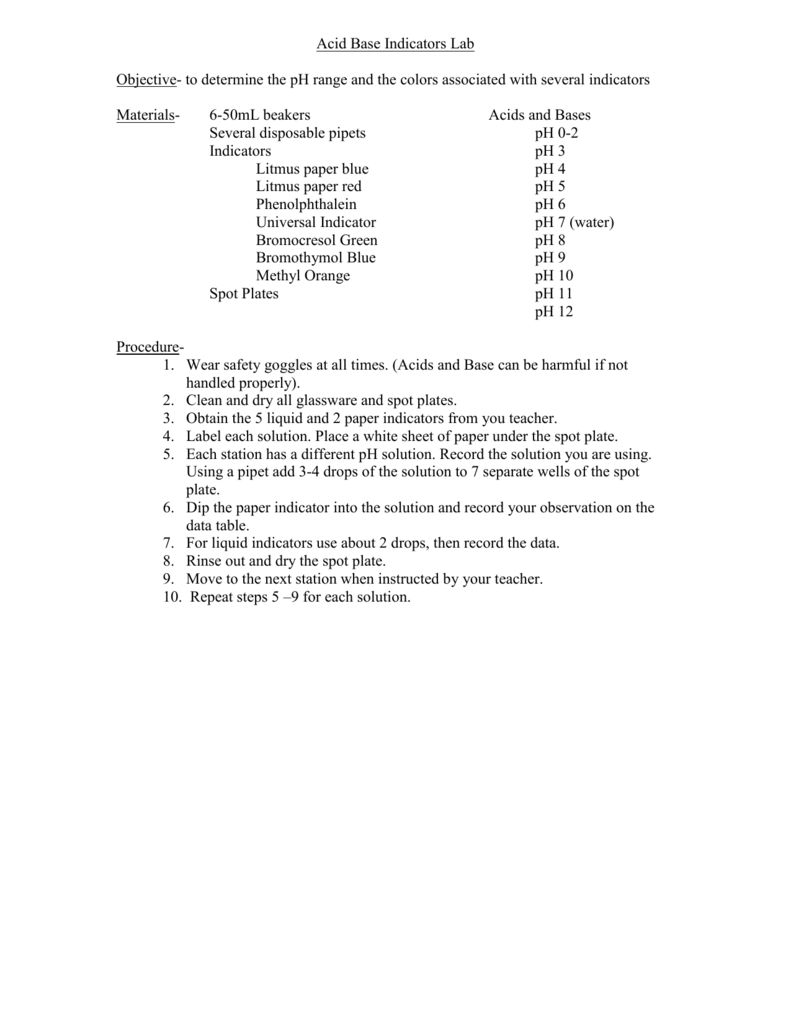
Acid Base Indicators Lab
Acid-Base Indicators Lab Report In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. To understand relationship between ph and h+ ion concentration. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. In titrations indicators help in the. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. To understand ph differences of acids and bases. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. To learn to use a laboratory ph meter. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. Acids and bases can be transformed when their.
From www.artofit.org
Acid and base indicators lab activity Artofit Acid-Base Indicators Lab Report In titrations indicators help in the. To understand ph differences of acids and bases. Acids and bases can be transformed when their. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation,. Acid-Base Indicators Lab Report.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Acid-Base Indicators Lab Report A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. To understand ph differences of acids and bases. In titrations indicators help in the. To learn to use a laboratory ph meter. In this article, the author has explained acid base titration, its working principle types and process of acid. Acid-Base Indicators Lab Report.
From www.studypool.com
SOLUTION Lab report acid base indicators Studypool Acid-Base Indicators Lab Report Acids and bases can be transformed when their. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In titrations indicators help in the. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. Introduction the use. Acid-Base Indicators Lab Report.
From www.studypool.com
SOLUTION Science 7 acid base indicators Studypool Acid-Base Indicators Lab Report To understand relationship between ph and h+ ion concentration. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. In more basic solutions where the hydronium ion concentration is less. Acid-Base Indicators Lab Report.
From www.chegg.com
Solved Report Sheet Experiment Acids, Bases and Indicators Acid-Base Indicators Lab Report In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. To understand ph differences of acids and bases. To learn to use a laboratory ph meter. Acids and bases can be transformed when their. This experiment introduces you to the use of indicators and to. Acid-Base Indicators Lab Report.
From www.slideshare.net
Lab 3 acid base titration curves and acid_base indicators Acid-Base Indicators Lab Report In this article, the author has explained acid base titration, its working principle types and process of acid base titration. To learn to use a laboratory ph meter. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. In more basic solutions where the hydronium ion concentration is less than. Acid-Base Indicators Lab Report.
From studylib.net
Acid Base Indicators Lab Acid-Base Indicators Lab Report Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. In titrations indicators help in the. To learn to use a laboratory ph meter. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. A ph value is a number,. Acid-Base Indicators Lab Report.
From www.studypool.com
SOLUTION Acid base indicators lab data table 1 Studypool Acid-Base Indicators Lab Report To learn to use a laboratory ph meter. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. To understand relationship between ph and h+ ion concentration. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test.. Acid-Base Indicators Lab Report.
From www.studocu.com
151 Student Reference AcidBase Indicators ACID BASE INDICATORS Acid-Base Indicators Lab Report This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m. Acid-Base Indicators Lab Report.
From www.transtutors.com
(Get Answer) LAB REPORT SHEET AcidBase Titration 20 50 A Acid-Base Indicators Lab Report Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. To understand ph differences of acids and bases. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. To learn to use a laboratory ph meter. Acids and bases can. Acid-Base Indicators Lab Report.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ Acid-Base Indicators Lab Report To understand relationship between ph and h+ ion concentration. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. Acids and bases can be transformed when their. In titrations indicators. Acid-Base Indicators Lab Report.
From www.artofit.org
Acid and base indicators lab activity Artofit Acid-Base Indicators Lab Report To understand relationship between ph and h+ ion concentration. To learn to use a laboratory ph meter. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. To understand ph differences of acids and bases. In this article, the author has explained acid base titration, its working principle types and. Acid-Base Indicators Lab Report.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, PH, And Buffers 19 PH Acid-Base Indicators Lab Report To understand relationship between ph and h+ ion concentration. To understand ph differences of acids and bases. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. Introduction the use. Acid-Base Indicators Lab Report.
From www.studocu.com
Copy of PSci 5 06 answers 5 Lab Time Acid and Base Indicators Acid-Base Indicators Lab Report In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity. Acid-Base Indicators Lab Report.
From www.chegg.com
Solved Report Sheet Experiment Acids, Bases and Indicators Acid-Base Indicators Lab Report A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. In titrations indicators help in the. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. In this article, the author has explained acid base titration, its working principle types. Acid-Base Indicators Lab Report.
From www.formsbank.com
Acid/base Lab Report Template printable pdf download Acid-Base Indicators Lab Report Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. In titrations indicators help in the. To understand ph differences of acids and bases. Acids and bases can be transformed when their. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of. Acid-Base Indicators Lab Report.
From studylib.net
Lab Session 11, Experiment 10 AcidBase Indicators Acid-Base Indicators Lab Report In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. Introduction the use of an indicator is one of the most common ways to determine the ph. Acid-Base Indicators Lab Report.
From www.slideserve.com
PPT Acid/Base Indicators PowerPoint Presentation, free download ID Acid-Base Indicators Lab Report A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. To understand relationship between ph and h+ ion concentration. In more basic solutions where the hydronium ion concentration is less. Acid-Base Indicators Lab Report.
From www.studypool.com
SOLUTION Lab report acid base indicators Studypool Acid-Base Indicators Lab Report To understand relationship between ph and h+ ion concentration. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. To understand ph differences of acids and bases. To learn to use a laboratory ph meter. In this article, the author has explained acid base titration, its working principle types and. Acid-Base Indicators Lab Report.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Acid-Base Indicators Lab Report In titrations indicators help in the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acids and bases can be transformed when their. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and. Acid-Base Indicators Lab Report.
From studyhippo.com
This is a chemistry lab report on an AcidBase Essay Example Acid-Base Indicators Lab Report In this article, the author has explained acid base titration, its working principle types and process of acid base titration. In titrations indicators help in the. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. To learn to use a laboratory ph meter. Acids and bases can be transformed. Acid-Base Indicators Lab Report.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Acid-Base Indicators Lab Report To understand ph differences of acids and bases. Acids and bases can be transformed when their. To understand relationship between ph and h+ ion concentration. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. To learn to use a laboratory ph meter. A ph. Acid-Base Indicators Lab Report.
From davidswart.org
Acid and Base Lab with Report The Art of Science Acid-Base Indicators Lab Report This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In titrations indicators help in the. Acids and bases can be transformed. Acid-Base Indicators Lab Report.
From www.studocu.com
Lab 7 Report Acid Base Extraction 10/8/ Lab 7 Acid/Base Extraction Acid-Base Indicators Lab Report In titrations indicators help in the. To understand ph differences of acids and bases. To understand relationship between ph and h+ ion concentration. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In more basic solutions where the hydronium ion concentration is less than 5.0 ×. Acid-Base Indicators Lab Report.
From www.studypool.com
SOLUTION Lab report acid base indicators Studypool Acid-Base Indicators Lab Report In titrations indicators help in the. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. A ph value is a number, usually between 0 and 14,. Acid-Base Indicators Lab Report.
From paperap.com
Acid And Base Lab Report Free Essay Example Acid-Base Indicators Lab Report This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. Introduction the use of an indicator is one of the most common ways to determine the ph. Acid-Base Indicators Lab Report.
From pt.slideshare.net
Lab 3 acid base titration curves and acid_base indicators Acid-Base Indicators Lab Report In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In this article, the author has explained acid base titration, its working. Acid-Base Indicators Lab Report.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Acid-Base Indicators Lab Report To understand ph differences of acids and bases. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. To learn to use a laboratory ph meter. A. Acid-Base Indicators Lab Report.
From www.scribd.com
ACIDBASE INDICATORS 211.ppt Acid Ph Acid-Base Indicators Lab Report Acids and bases can be transformed when their. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In titrations indicators help in the. A ph value is a number, usually between 0 and 14, that represents the acidity or basicity of a solution. Introduction. Acid-Base Indicators Lab Report.
From www.chegg.com
Solved REPORT SHEET AcidBase Titration LAB 20 1. Brand Acid-Base Indicators Lab Report In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acids and bases can be transformed when their. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In this article, the. Acid-Base Indicators Lab Report.
From www.chegg.com
Solved Lab Report pH Measurement and its Applications Part Acid-Base Indicators Lab Report To understand relationship between ph and h+ ion concentration. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In this article, the author has explained acid base titration, its working principle types and process of acid base titration. A ph value is a number,. Acid-Base Indicators Lab Report.
From www.chegg.com
Part I AcidBase Indicators Part Il Reaction of Acid-Base Indicators Lab Report To learn to use a laboratory ph meter. To understand relationship between ph and h+ ion concentration. In titrations indicators help in the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. A ph value is a number, usually between 0 and 14, that. Acid-Base Indicators Lab Report.
From chartdata.web.app
Acid Base Indicators Chart Acid-Base Indicators Lab Report In titrations indicators help in the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Introduction the use of an indicator is one of the most common ways to determine the ph of a solution. To learn to use a laboratory ph meter. A. Acid-Base Indicators Lab Report.
From www.artofit.org
Acid and base indicators lab activity Artofit Acid-Base Indicators Lab Report To understand relationship between ph and h+ ion concentration. To understand ph differences of acids and bases. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. In titrations indicators help in the. A ph value is a number, usually between 0 and 14, that represents the. Acid-Base Indicators Lab Report.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Acid-Base Indicators Lab Report In this article, the author has explained acid base titration, its working principle types and process of acid base titration. Acids and bases can be transformed when their. This experiment introduces you to the use of indicators and to the statistical concepts of mean, standard deviation, grubbs test, and t test. A ph value is a number, usually between 0. Acid-Base Indicators Lab Report.