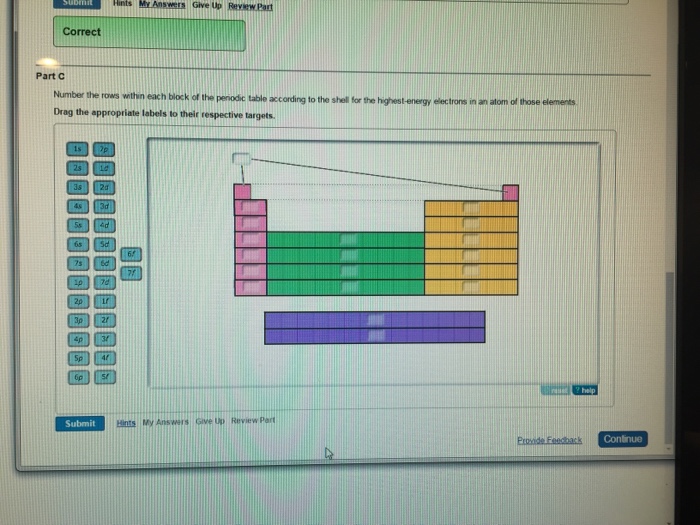
Shell Energy Block Number . Each shell has a different energy level, increasing the further it is from the nucleus. The rule to calculate the number of electrons that each shell can hold is 2n 2. In any atom with two or more electrons, the repulsion between the electrons makes. Electrons in the same shell have the same. Electrons orbit the nucleus of an atom at different ranges, called shells. In the diagram above the energy. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. The number of elements in each block is the same as in the energy level it corresponds. There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The first shell is 2 (1) 2 which gives you 2 electrons. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). Each energy level is given a. The first shell can only contain two electrons, the second eight and the third eighteen.
from www.chegg.com
There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. In the diagram above the energy. The number of elements in each block is the same as in the energy level it corresponds. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. In any atom with two or more electrons, the repulsion between the electrons makes. The first shell can only contain two electrons, the second eight and the third eighteen. Electrons in the same shell have the same. Electrons orbit the nucleus of an atom at different ranges, called shells. Each energy level is given a. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n).
Solved Number the rows within each block of the periodic
Shell Energy Block Number The first shell is 2 (1) 2 which gives you 2 electrons. Electrons in the same shell have the same. The first shell can only contain two electrons, the second eight and the third eighteen. In any atom with two or more electrons, the repulsion between the electrons makes. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. The rule to calculate the number of electrons that each shell can hold is 2n 2. Electrons orbit the nucleus of an atom at different ranges, called shells. In the diagram above the energy. There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The first shell is 2 (1) 2 which gives you 2 electrons. Each shell has a different energy level, increasing the further it is from the nucleus. The number of elements in each block is the same as in the energy level it corresponds. Each energy level is given a.
From www.shell.com.ph
Shell Mamplasan operations to be powered by renewable energy Shell Shell Energy Block Number Each energy level is given a. Electrons orbit the nucleus of an atom at different ranges, called shells. The first shell is 2 (1) 2 which gives you 2 electrons. In the diagram above the energy. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. The number of elements in each block is the same as. Shell Energy Block Number.
From fakedocshop.com
Shell Energy Electricity And Gas Bill PDF Template Shell Energy Block Number The first shell is 2 (1) 2 which gives you 2 electrons. The first shell can only contain two electrons, the second eight and the third eighteen. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. Each shell has a different energy level, increasing the further it is from the nucleus. Electrons in the same shell. Shell Energy Block Number.
From www.chegg.com
Solved Number the rows within each block of the periodic Shell Energy Block Number Each energy level is given a. In any atom with two or more electrons, the repulsion between the electrons makes. Electrons orbit the nucleus of an atom at different ranges, called shells. Each shell has a different energy level, increasing the further it is from the nucleus. Electrons in the same shell have the same. In the diagram above the. Shell Energy Block Number.
From futurefuels.blog
Energy Transition Strategy Shell will bis 2050 klimaneutral sein Shell Energy Block Number Each energy level is given a. The rule to calculate the number of electrons that each shell can hold is 2n 2. Electrons in the same shell have the same. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. Electrons orbit the nucleus of an atom at different ranges, called shells. Each shell has a different. Shell Energy Block Number.
From shellenergy.com
How to read your residential Texas electricity bill? Shell Energy Shell Energy Block Number The first shell is 2 (1) 2 which gives you 2 electrons. The first shell can only contain two electrons, the second eight and the third eighteen. Each energy level is given a. Each shell has a different energy level, increasing the further it is from the nucleus. In any atom with two or more electrons, the repulsion between the. Shell Energy Block Number.
From www.reporter.gr
Τρεις προσφορές για τη Shell Energy στη Βρετανία Shell Energy Block Number Each energy level is given a. There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The first shell is 2 (1) 2 which gives you 2 electrons. Electrons in the same shell have the same. The rule to calculate the number of electrons that. Shell Energy Block Number.
From www.shell.co.jp
メディアセンター Shell 日本 Shell Energy Block Number Electrons orbit the nucleus of an atom at different ranges, called shells. In the diagram above the energy. Each energy level is given a. The first shell is 2 (1) 2 which gives you 2 electrons. There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of. Shell Energy Block Number.
From butchixanh.edu.vn
Periodic Table & Energy Levels (Electrons per shell) Bút Chì Xanh Shell Energy Block Number Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). In any atom with two or more electrons, the repulsion between the electrons makes. Each shell has a different energy level, increasing the further it is from the nucleus. The rule to calculate the number of electrons that each shell can hold. Shell Energy Block Number.
From login.webmail.shellenergy.co.uk
Log in Shell Energy Shell Energy Block Number The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. In the diagram above the energy. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period. Shell Energy Block Number.
From www.shell.in
Shell Energy Marketing & Trading Shell India Shell Energy Block Number The rule to calculate the number of electrons that each shell can hold is 2n 2. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). The number of elements in each block is the same as in the energy level it corresponds. Electrons in the same shell have the same. Each. Shell Energy Block Number.
From www.expertreviews.co.uk
Broadband deals Get LUDICROUS discounts from Shell Energy Expert Reviews Shell Energy Block Number There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The number of elements in each block is the same as in the energy level it corresponds. Electrons orbit the nucleus of an atom at different ranges, called shells. In the diagram above the energy.. Shell Energy Block Number.
From ariasigns.com
New and Improved Shell Energy Stadium Aria Signs & Design Shell Energy Block Number In the diagram above the energy. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. Each energy level is given a. There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. Electrons orbit the nucleus of an atom at different ranges,. Shell Energy Block Number.
From www.youtube.com
TOP 6 SHELL ENERGY BLACK FRIDAY BROADBAND DEALS FROM 6TH NOVEMBER Shell Energy Block Number Each energy level is given a. The number of elements in each block is the same as in the energy level it corresponds. In any atom with two or more electrons, the repulsion between the electrons makes. In the diagram above the energy. Electrons in the same shell have the same. The first shell can only contain two electrons, the. Shell Energy Block Number.
From www.independent.co.uk
Shell plans strategic review of energy supply business which employs Shell Energy Block Number Each shell has a different energy level, increasing the further it is from the nucleus. Electrons in the same shell have the same. The number of elements in each block is the same as in the energy level it corresponds. The rule to calculate the number of electrons that each shell can hold is 2n 2. Electrons orbit the nucleus. Shell Energy Block Number.
From intempl.com
UNITED KINGDOM SHELL ENERGY utility bill template Word and PDF template Shell Energy Block Number In any atom with two or more electrons, the repulsion between the electrons makes. Electrons orbit the nucleus of an atom at different ranges, called shells. Each energy level is given a. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). Electrons in the same shell have the same. The energy. Shell Energy Block Number.
From www.marketbeat.com
Park Avenue Securities LLC Buys 16,317 Shares of Shell plc (NYSESHEL Shell Energy Block Number Each energy level is given a. The number of elements in each block is the same as in the energy level it corresponds. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). The rule to calculate the number of electrons that each shell can hold is 2n 2. The first shell. Shell Energy Block Number.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Shell Energy Block Number Each shell has a different energy level, increasing the further it is from the nucleus. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). In any atom with two or more electrons, the repulsion between the electrons makes. There's an important distinction between the number of electrons possible in a shell. Shell Energy Block Number.
From www.linkedin.com
Shell Energy on LinkedIn Broker Reward Trip 2023 Shell Energy Block Number Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). The first shell can only contain two electrons, the second eight and the third eighteen. In any atom with two or more electrons, the repulsion between the electrons makes. There's an important distinction between the number of electrons possible in a shell. Shell Energy Block Number.
From appadvice.com
Shell Energy by Shell Energy Retail Limited Shell Energy Block Number In any atom with two or more electrons, the repulsion between the electrons makes. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. The first shell can only contain two electrons, the second eight and the third eighteen. In the diagram above the energy. Electrons in the same shell have the same. Electrons are organized according. Shell Energy Block Number.
From www.chegg.com
Solved Number the rows within each block of the periodic Shell Energy Block Number In the diagram above the energy. There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The first shell is 2 (1) 2 which gives you 2 electrons. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. Electrons orbit the nucleus. Shell Energy Block Number.
From www.moneysavingexpert.com
Two million Shell energy and broadband customers could be affected as Shell Energy Block Number The number of elements in each block is the same as in the energy level it corresponds. Each shell has a different energy level, increasing the further it is from the nucleus. Electrons orbit the nucleus of an atom at different ranges, called shells. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum. Shell Energy Block Number.
From jp.cointelegraph.com
住友商事とシェル、米LO3エナジーに出資 ブロックチェーンを用いた電力プラットフォーム事業進める Cointelegraph コイン Shell Energy Block Number Each shell has a different energy level, increasing the further it is from the nucleus. Each energy level is given a. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. The first shell is 2 (1) 2 which gives you 2 electrons. The first shell can only contain two electrons, the second eight and the third. Shell Energy Block Number.
From www.techradar.com
Shell Energy review TechRadar Shell Energy Block Number There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The first shell is 2 (1) 2 which gives you 2 electrons. The first shell can only contain two electrons, the second eight and the third eighteen. In the diagram above the energy. The rule. Shell Energy Block Number.
From www.shell.com
Shell Energy Europe Shell Global Shell Energy Block Number There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The rule to calculate the number of electrons that each shell can hold is 2n 2. The first shell is 2 (1) 2 which gives you 2 electrons. In the diagram above the energy. Electrons. Shell Energy Block Number.
From abraceel.com.br
Shell Energy Brasil ABRACEEL Shell Energy Block Number The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. The number of elements in each block is the same as in the energy level it corresponds. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). In the diagram above the energy. Electrons in the same shell have. Shell Energy Block Number.
From www.inventiva.co.in
Shell Energy India To Invest Rs 3500 Crore In Gujarat Inventiva Shell Energy Block Number Electrons in the same shell have the same. The first shell is 2 (1) 2 which gives you 2 electrons. In the diagram above the energy. Electrons orbit the nucleus of an atom at different ranges, called shells. The first shell can only contain two electrons, the second eight and the third eighteen. The number of elements in each block. Shell Energy Block Number.
From www.chegg.com
Solved Number the rows within each block of the periodic Shell Energy Block Number Electrons in the same shell have the same. Electrons orbit the nucleus of an atom at different ranges, called shells. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. The number of elements in each block is the same as in the energy level it corresponds. The first shell is 2 (1) 2 which gives you. Shell Energy Block Number.
From www.upi.com
Shell focused on energy transition, but spending big on fossil fuels Shell Energy Block Number The first shell can only contain two electrons, the second eight and the third eighteen. The rule to calculate the number of electrons that each shell can hold is 2n 2. The number of elements in each block is the same as in the energy level it corresponds. Electrons orbit the nucleus of an atom at different ranges, called shells.. Shell Energy Block Number.
From unacademy.com
Notes on the Irregularites of Electronic Configuration of Transition Metals Shell Energy Block Number The rule to calculate the number of electrons that each shell can hold is 2n 2. Each shell has a different energy level, increasing the further it is from the nucleus. In the diagram above the energy. Electrons orbit the nucleus of an atom at different ranges, called shells. Each energy level is given a. In any atom with two. Shell Energy Block Number.
From www.brokeronlinexchange.com
February Supplier of the Month Shell Energy Broker Online Exchange Shell Energy Block Number The number of elements in each block is the same as in the energy level it corresponds. Each energy level is given a. Electrons orbit the nucleus of an atom at different ranges, called shells. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). The energy of atomic orbitals increases as. Shell Energy Block Number.
From www.ourenergypolicy.org
Shell Energy Transition Progress Report 2022 OurEnergyPolicy Shell Energy Block Number In the diagram above the energy. The number of elements in each block is the same as in the energy level it corresponds. The rule to calculate the number of electrons that each shell can hold is 2n 2. The first shell is 2 (1) 2 which gives you 2 electrons. Electrons are organized according to their energies into sets. Shell Energy Block Number.
From ariasigns.com
New and Improved Shell Energy Stadium Aria Signs & Design Shell Energy Block Number Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). There's an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. Each energy level is given a. The first shell is 2 (1) 2 which gives you 2. Shell Energy Block Number.
From theskipper.ie
Shell Leads Global Lubricants Market For 16ᵗʰ Year The Skipper Shell Energy Block Number Electrons in the same shell have the same. Electrons orbit the nucleus of an atom at different ranges, called shells. In any atom with two or more electrons, the repulsion between the electrons makes. The first shell is 2 (1) 2 which gives you 2 electrons. Electrons are organized according to their energies into sets called shells (labeled by the. Shell Energy Block Number.
From shellenergy.com
How to read your residential Texas electricity bill? Shell Energy Shell Energy Block Number The first shell can only contain two electrons, the second eight and the third eighteen. In any atom with two or more electrons, the repulsion between the electrons makes. Each shell has a different energy level, increasing the further it is from the nucleus. There's an important distinction between the number of electrons possible in a shell and the number. Shell Energy Block Number.
From www.techradar.com
Shell Energy's Superfast Fibre Plus broadband is now only £25.99 per Shell Energy Block Number In any atom with two or more electrons, the repulsion between the electrons makes. Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. Each shell has a different energy level, increasing the further it is from the nucleus.. Shell Energy Block Number.