Does Water Pressure Affect Water Temperature . yes, at constant density, the pressure increases as the temperature does: the change in specific volume for a given change in temperature is not the same at various beginning temperatures. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. This is the triple point. if you decrease the pressure, the freezing point of water will increase ever so slightly. From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0.
from www.alamy.com
This is the triple point. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. yes, at constant density, the pressure increases as the temperature does: the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. if you decrease the pressure, the freezing point of water will increase ever so slightly. the change in specific volume for a given change in temperature is not the same at various beginning temperatures.
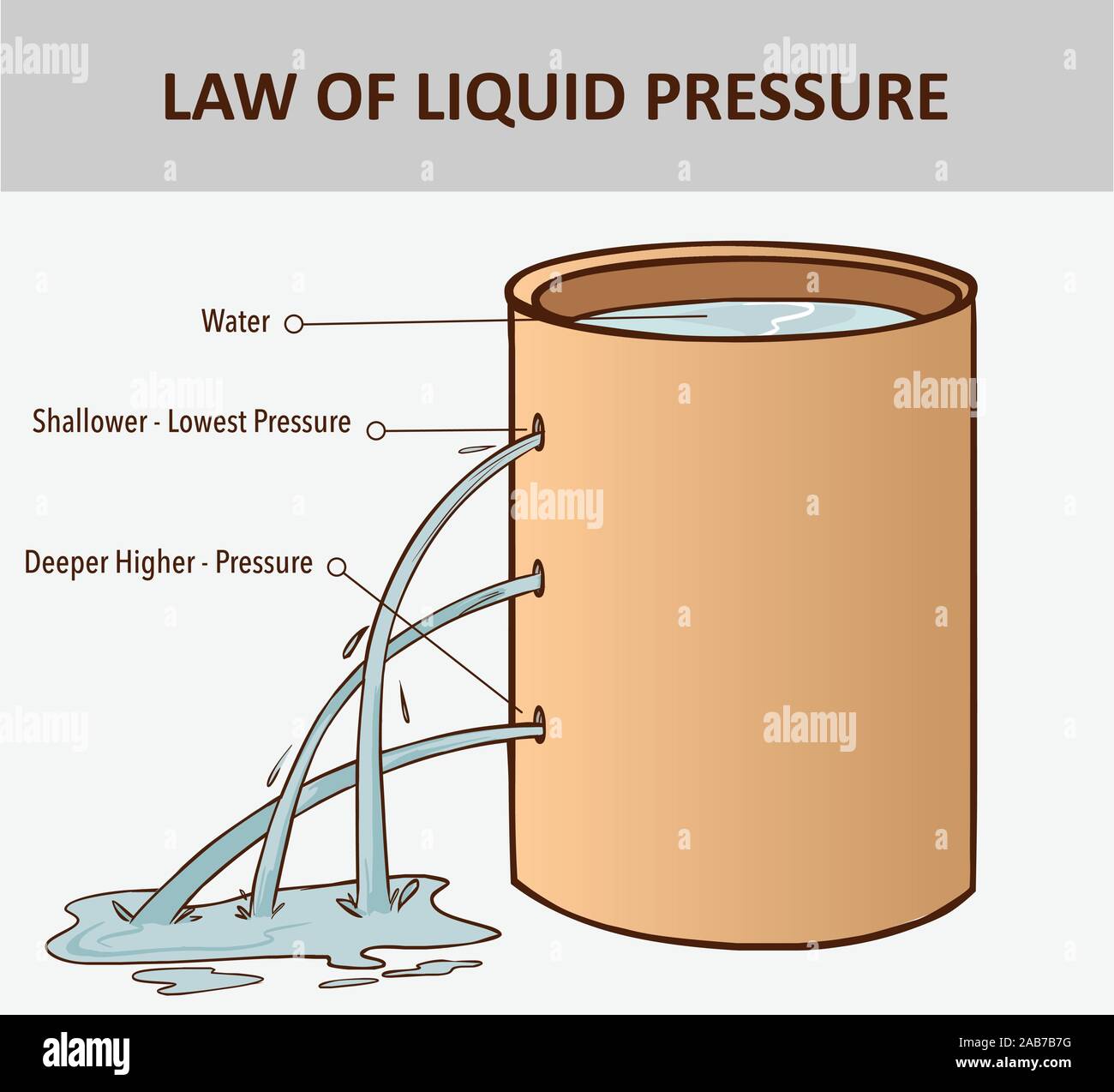
Pressure in water. The pressure in a liquid increases with depth. Liquids pressure. Ocean
Does Water Pressure Affect Water Temperature From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. if you decrease the pressure, the freezing point of water will increase ever so slightly. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. This is the triple point. From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. yes, at constant density, the pressure increases as the temperature does:
From heaterguides.com
Does Water Heater Affect Water Pressure? Does Water Pressure Affect Water Temperature the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. This is the triple point. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. yes, at constant density, the pressure increases as the temperature. Does Water Pressure Affect Water Temperature.
From mydiagram.online
[DIAGRAM] Pressure Vs Temperature Phase Diagram For Water Does Water Pressure Affect Water Temperature the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. if you decrease the pressure, the freezing point of water will increase ever so. Does Water Pressure Affect Water Temperature.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube Does Water Pressure Affect Water Temperature the change in specific volume for a given change in temperature is not the same at various beginning temperatures. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. if you decrease the pressure, the freezing point of water will increase. Does Water Pressure Affect Water Temperature.
From mydiagram.online
[DIAGRAM] Pressure Temperature Phase Diagram For Water Does Water Pressure Affect Water Temperature if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing. Does Water Pressure Affect Water Temperature.
From www.engineeringtoolbox.com
Water Saturation Pressure vs. Temperature Does Water Pressure Affect Water Temperature the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. This is the triple point. the density of water increases with decreasing temperature, reaching. Does Water Pressure Affect Water Temperature.
From www.alamy.com
Pressure in water. The pressure in a liquid increases with depth. Liquids pressure. Ocean Does Water Pressure Affect Water Temperature the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. if you decrease the pressure, the freezing point of water will increase ever so slightly. From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm.. Does Water Pressure Affect Water Temperature.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Does Water Pressure Affect Water Temperature From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. This is the triple point. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases. Does Water Pressure Affect Water Temperature.
From www.slideserve.com
PPT Fluid Mechanics PowerPoint Presentation, free download ID6711078 Does Water Pressure Affect Water Temperature the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. if you decrease the pressure, the freezing point of water will increase ever so slightly. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point. Does Water Pressure Affect Water Temperature.
From www.youtube.com
Properties of Water How Does a Temperature Affect Water Molecules? YouTube Does Water Pressure Affect Water Temperature the change in specific volume for a given change in temperature is not the same at various beginning temperatures. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. if you decrease the pressure, the freezing point of water will increase ever so slightly.. Does Water Pressure Affect Water Temperature.
From www.expresssewer.com
How Does Water Pressure and Flow Work? Does Water Pressure Affect Water Temperature yes, at constant density, the pressure increases as the temperature does: the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. From 0° c at. Does Water Pressure Affect Water Temperature.
From www.tec-science.com
Pressure in liquids (hydrostatic pressure) tecscience Does Water Pressure Affect Water Temperature yes, at constant density, the pressure increases as the temperature does: if you decrease the pressure, the freezing point of water will increase ever so slightly. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. From 0° c at 1. Does Water Pressure Affect Water Temperature.
From serc.carleton.edu
The Chemistry of Natural Waters Does Water Pressure Affect Water Temperature From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. This is the triple point. yes, at constant density, the pressure increases as the temperature does: the change in specific volume for a given change in temperature is not the same at various beginning temperatures. the melting point of water. Does Water Pressure Affect Water Temperature.
From mavink.com
Water Vapour Pressure Chart Bar Does Water Pressure Affect Water Temperature the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. if you decrease the pressure, the freezing point of water will increase ever so slightly. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about. Does Water Pressure Affect Water Temperature.
From chart-studio.plotly.com
Water Pressure vs. Temperature line chart made by 18youngt plotly Does Water Pressure Affect Water Temperature the change in specific volume for a given change in temperature is not the same at various beginning temperatures. yes, at constant density, the pressure increases as the temperature does: This is the triple point. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature. Does Water Pressure Affect Water Temperature.
From www.sciencelearn.org.nz
Ocean salinity, temperature and density — Science Learning Hub Does Water Pressure Affect Water Temperature the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. yes, at constant density, the pressure increases as the temperature does: the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature. Does Water Pressure Affect Water Temperature.
From www.askmattrab.com
Pressure Liquid Pressure, Pascal's Law and Class Ten Science Does Water Pressure Affect Water Temperature if you decrease the pressure, the freezing point of water will increase ever so slightly. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. This is the triple point. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as. Does Water Pressure Affect Water Temperature.
From www.tffn.net
Understanding How Water Pressure Works Exploring the Physics, Troubleshooting, and Boosting Does Water Pressure Affect Water Temperature if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. the melting point of water is dependent of the pressure above the ice (solid water), and the melting point or freezing temperature decreases with increasing pressure. This is the triple point. the density of water increases with decreasing temperature, reaching. Does Water Pressure Affect Water Temperature.
From www.thespruce.com
A Homeowner's Guide to Water Pressure Does Water Pressure Affect Water Temperature yes, at constant density, the pressure increases as the temperature does: if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. This is the triple point. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. the density of water. Does Water Pressure Affect Water Temperature.
From pressbooks.online.ucf.edu
9.4 Mixtures of Gases and Partial Pressures Chemistry Fundamentals Does Water Pressure Affect Water Temperature the change in specific volume for a given change in temperature is not the same at various beginning temperatures. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. This is the triple point. yes, at constant density, the pressure increases as the temperature does: the density of water. Does Water Pressure Affect Water Temperature.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Does Water Pressure Affect Water Temperature the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. if you decrease the pressure, the freezing point of water will increase ever so slightly.. Does Water Pressure Affect Water Temperature.
From www.slideserve.com
PPT Chapter 13 Fluids PowerPoint Presentation ID350101 Does Water Pressure Affect Water Temperature This is the triple point. if you decrease the pressure, the freezing point of water will increase ever so slightly. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. From 0° c at 1 atm pressure it will increase up to 0.01° c at. Does Water Pressure Affect Water Temperature.
From chem.libretexts.org
13.4 Pressure and Temperature Effects on Solubility Chemistry LibreTexts Does Water Pressure Affect Water Temperature the change in specific volume for a given change in temperature is not the same at various beginning temperatures. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. the melting point of water is dependent of the pressure above the ice (solid water),. Does Water Pressure Affect Water Temperature.
From www3.epa.gov
Climate Impacts on Water Resources Climate Change US EPA Does Water Pressure Affect Water Temperature if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. yes, at constant density, the pressure increases as the temperature does: if you decrease the pressure, the freezing point of water will increase ever so slightly. the change in specific volume for a given change in temperature is not. Does Water Pressure Affect Water Temperature.
From www.researchgate.net
Relationship between temperature and surface tension of liquid water. Download Scientific Diagram Does Water Pressure Affect Water Temperature the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. the melting point of water is dependent of the pressure above the ice (solid water), and the. Does Water Pressure Affect Water Temperature.
From www.michiganseagrant.org
Properties of Water Teaching Great Lakes Science Does Water Pressure Affect Water Temperature This is the triple point. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. if you decrease the pressure, the freezing point of water. Does Water Pressure Affect Water Temperature.
From www.slideserve.com
PPT 22 Properties of water PowerPoint Presentation ID379006 Does Water Pressure Affect Water Temperature if you decrease the pressure, the freezing point of water will increase ever so slightly. This is the triple point. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. yes, at constant density, the pressure increases as the temperature does: the melting point of water is dependent of. Does Water Pressure Affect Water Temperature.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Experiments Does Water Pressure Affect Water Temperature if you decrease the pressure, the freezing point of water will increase ever so slightly. yes, at constant density, the pressure increases as the temperature does: This is the triple point. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. From 0° c. Does Water Pressure Affect Water Temperature.
From atlas-scientific.com
How Does Temperature Affect Dissolved Oxygen? Atlas Scientific Does Water Pressure Affect Water Temperature if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. if you decrease the pressure, the freezing point of water will increase ever so slightly. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. This is the triple point. . Does Water Pressure Affect Water Temperature.
From lessonlibspelunking.z22.web.core.windows.net
What Happens To Ph When Temperature Increases Does Water Pressure Affect Water Temperature if you decrease the pressure, the freezing point of water will increase ever so slightly. yes, at constant density, the pressure increases as the temperature does: if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. This is the triple point. From 0° c at 1 atm pressure it will. Does Water Pressure Affect Water Temperature.
From mavink.com
Water Flow Vs Pressure Chart Does Water Pressure Affect Water Temperature From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. if you decrease the pressure, the freezing point of water will increase ever so slightly. the melting. Does Water Pressure Affect Water Temperature.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning Industrial Heat Does Water Pressure Affect Water Temperature if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. This is the triple point. if you decrease the pressure, the freezing point of water will increase. Does Water Pressure Affect Water Temperature.
From exorpxhjd.blob.core.windows.net
Evaporation Vapor Pressure Of Water at Marcus Quigley blog Does Water Pressure Affect Water Temperature if you decrease the pressure, the freezing point of water will increase ever so slightly. From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. the melting point of water is dependent of. Does Water Pressure Affect Water Temperature.
From www.quora.com
How does the increase in temperature affect water pressure? Quora Does Water Pressure Affect Water Temperature yes, at constant density, the pressure increases as the temperature does: the change in specific volume for a given change in temperature is not the same at various beginning temperatures. the density of water increases with decreasing temperature, reaching a maximum at 4.0 °c, and then decreases as the temperature falls below 4.0. the melting point. Does Water Pressure Affect Water Temperature.
From uooz.com
Home Water Pressure Everything A Homeowner Should Know! Does Water Pressure Affect Water Temperature From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. if you raise the pressure keeping the temperature constant, it'll switch to $\color{blue}{\textbf{ice vi}}$ at about 1gpa,. the density of water. Does Water Pressure Affect Water Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Water Pressure Affect Water Temperature This is the triple point. From 0° c at 1 atm pressure it will increase up to 0.01° c at 0.006 atm. the change in specific volume for a given change in temperature is not the same at various beginning temperatures. if you decrease the pressure, the freezing point of water will increase ever so slightly. if. Does Water Pressure Affect Water Temperature.