Endothermic Reaction Lab Experiment . Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Next, students explore the relationship between an observed change in temperature and the. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. In this investigation, students classify chemical reactions as exothermic or endothermic. These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Other chemical reactions release energy in the form of heat, light, or sound. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic.
from vhmsscience.weebly.com
In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. In this investigation, students classify chemical reactions as exothermic or endothermic. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Next, students explore the relationship between an observed change in temperature and the. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic.
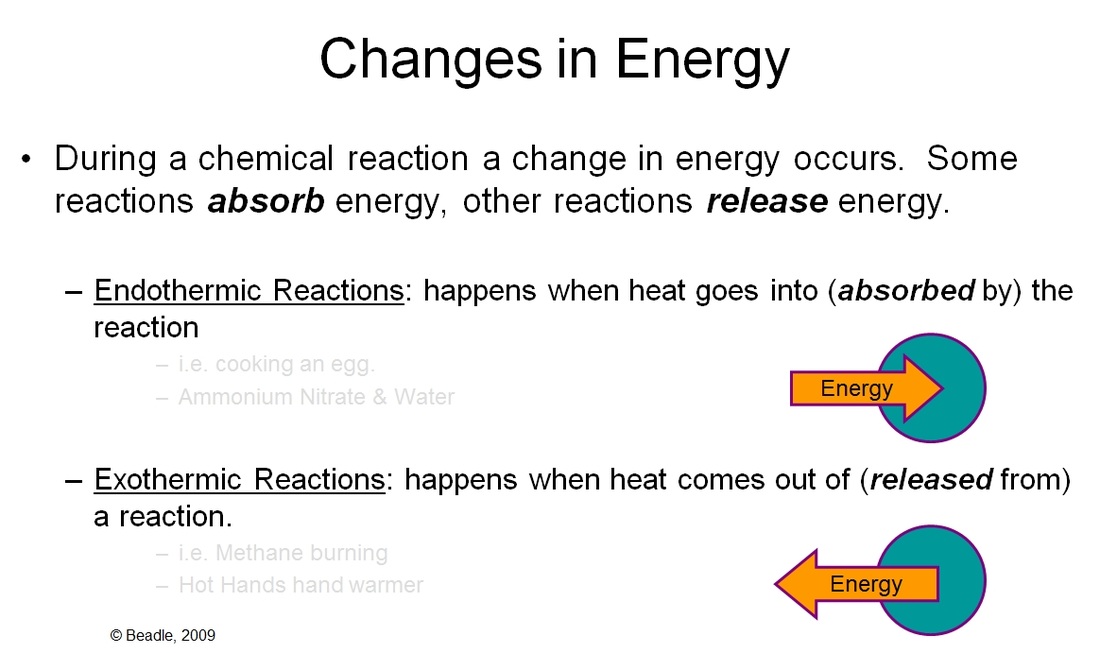
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE
Endothermic Reaction Lab Experiment Other chemical reactions release energy in the form of heat, light, or sound. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. In this investigation, students classify chemical reactions as exothermic or endothermic. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Next, students explore the relationship between an observed change in temperature and the. Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Lab Experiment In this investigation, students classify chemical reactions as exothermic or endothermic. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Not all endothermic reactions are safe to touch, so their. Endothermic Reaction Lab Experiment.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Lab Experiment This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Not all endothermic reactions are safe to. Endothermic Reaction Lab Experiment.
From www.youtube.com
Endothermic Reaction, SMESES Lab YouTube Endothermic Reaction Lab Experiment Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Other chemical reactions release energy in the. Endothermic Reaction Lab Experiment.
From www.youtube.com
Pre Lab Video Experiment 1 Endothermic and Exothermic Reactions YouTube Endothermic Reaction Lab Experiment In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. In this investigation, students classify chemical reactions as exothermic or endothermic. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Learn about endothermic and exothermic reactions and energy. Endothermic Reaction Lab Experiment.
From www.vernier.com
Endothermic and Exothermic Reactions > Experiment 5 from Physical Endothermic Reaction Lab Experiment Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). These are instructions for a safe and. Endothermic Reaction Lab Experiment.
From www.vernier.com
Endothermic and Exothermic Reactions > Experiment 1 from Chemistry with Endothermic Reaction Lab Experiment This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. In. Endothermic Reaction Lab Experiment.
From www.pinterest.com
For this Endothermic and Exothermic Reactions Lab, students will Endothermic Reaction Lab Experiment In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Next, students explore the relationship between an observed change in temperature and the. Other chemical reactions release energy in the form of heat, light, or sound. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces. Endothermic Reaction Lab Experiment.
From studylib.net
Exothermic and Endothermic Reactions Lab Endothermic Reaction Lab Experiment This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic.. Endothermic Reaction Lab Experiment.
From www.youtube.com
AN ENDOTHERMIC REACTION EXPERIMENT YouTube Endothermic Reaction Lab Experiment Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Next, students explore the relationship between an observed change in temperature and the. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. In this investigation, students classify. Endothermic Reaction Lab Experiment.
From frugalfun4boys.com
Chilly Chemical Reactions! {Endothermic Reactions} Frugal Fun For Endothermic Reaction Lab Experiment This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. In. Endothermic Reaction Lab Experiment.
From fity.club
Endothermic And Exothermic Lab Endothermic Reaction Lab Experiment Next, students explore the relationship between an observed change in temperature and the. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Other chemical reactions release energy in the form of heat, light, or sound. Endothermic reactions are characterized by positive heat flow (into the reaction) and an. Endothermic Reaction Lab Experiment.
From www.pinterest.com
Exothermic or Endothermic Reaction Chemical Reactions NGSS MSPS12 Endothermic Reaction Lab Experiment Other chemical reactions release energy in the form of heat, light, or sound. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Endothermic reactions are characterized by positive heat flow (into the reaction). Endothermic Reaction Lab Experiment.
From www.studocu.com
Endothermic and Exothermic Reactions with lab notebook report Endothermic Reaction Lab Experiment Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Not all endothermic reactions. Endothermic Reaction Lab Experiment.
From www.pinterest.co.uk
Endothermic Reactions Chemistry classroom, Homeschool science Endothermic Reaction Lab Experiment In this investigation, students classify chemical reactions as exothermic or endothermic. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Students measure the temperature changes in different reactions taking place. Endothermic Reaction Lab Experiment.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Lab Experiment Next, students explore the relationship between an observed change in temperature and the. Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Other chemical reactions release energy in the form of heat, light, or sound. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling. Endothermic Reaction Lab Experiment.
From proper-cooking.info
Endothermic And Exothermic Reaction Experiment Endothermic Reaction Lab Experiment Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Other chemical reactions release energy in the form of heat, light, or sound. Endothermic reactions are characterized by. Endothermic Reaction Lab Experiment.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Lab Experiment Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. In this investigation, students classify chemical reactions as exothermic or endothermic. Endothermic reactions are characterized by positive heat flow (into the. Endothermic Reaction Lab Experiment.
From www.pinterest.com
Endothermic and Exothermic Reactions Lab ⋆ Chemistry Endothermic Reaction Lab Experiment Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. In this investigation, students classify chemical reactions as exothermic or endothermic. Other chemical reactions release energy in the form of heat, light, or sound. Learn. Endothermic Reaction Lab Experiment.
From www.fizzicseducation.com.au
Endothermic reaction Fizzics Education Endothermic Reaction Lab Experiment Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Exothermic reactions may occur spontaneously and result in higher. Endothermic Reaction Lab Experiment.
From teachsimple.com
Baggie Chemistry Lab Endothermic and Exothermic Reactions by Teach Simple Endothermic Reaction Lab Experiment Next, students explore the relationship between an observed change in temperature and the. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. Other chemical reactions release energy in the form of heat, light,. Endothermic Reaction Lab Experiment.
From www.youtube.com
ENDOTHERMIC AND EXOTHERMIC REACTIONS/ LABORATORY EXPERIMENT YouTube Endothermic Reaction Lab Experiment Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer.. Endothermic Reaction Lab Experiment.
From teachingscience.us
Exothermic and Endothermic Reactions Endothermic Reaction Lab Experiment Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Next, students explore the relationship between an observed change in temperature and the. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. This simple chemistry demonstration is an endothermic reaction. Endothermic Reaction Lab Experiment.
From www.pinterest.com
Endothermic & Exothermic Reactions Stations Exothermic reaction Endothermic Reaction Lab Experiment Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). In this investigation, students classify chemical reactions as exothermic or endothermic. Next, students explore the relationship between an observed change in temperature and the. These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Exothermic reactions. Endothermic Reaction Lab Experiment.
From www.pinterest.com.au
Endothermic or Exothermic Reaction? Science experiments, Experiments Endothermic Reaction Lab Experiment These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Other chemical reactions release energy in the form of heat, light, or sound. In this demonstration or class experiment, students observe an endothermic reaction between. Endothermic Reaction Lab Experiment.
From www.fizzicseducation.com.au
Endothermic reaction Fizzics Education Endothermic Reaction Lab Experiment Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. In this investigation, students classify chemical reactions. Endothermic Reaction Lab Experiment.
From www.fizzicseducation.com.au
Endothermic reaction science experiment Fizzics Education Endothermic Reaction Lab Experiment Other chemical reactions release energy in the form of heat, light, or sound. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Next, students explore the relationship between an observed change in temperature and. Endothermic Reaction Lab Experiment.
From labonlaptop.com
Learn ExothermicEndothermic chemical reactions labOnLaptop Store Endothermic Reaction Lab Experiment In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Other chemical reactions release energy in the form of heat, light, or sound. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. In this investigation, students classify chemical. Endothermic Reaction Lab Experiment.
From www.scribd.com
Exothermic Endothermic Reaction Lab PDF Catalase Properties Of Water Endothermic Reaction Lab Experiment In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Learn about. Endothermic Reaction Lab Experiment.
From teachingscience.us
Exothermic and Endothermic Reactions Middle School Science Unit Endothermic Reaction Lab Experiment Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Not all endothermic reactions are safe to. Endothermic Reaction Lab Experiment.
From teachingscience.us
Exothermic and Endothermic Reactions Endothermic Reaction Lab Experiment Next, students explore the relationship between an observed change in temperature and the. This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. These are instructions for a safe and easy endothermic chemical reaction demonstration that uses common, nontoxic ingredients. Other chemical reactions release energy in the form of. Endothermic Reaction Lab Experiment.
From frugalfun4boys.com
Chilly Chemical Reactions! {Endothermic Reactions} Frugal Fun For Endothermic Reaction Lab Experiment Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in. Other chemical reactions release energy in the form of heat,. Endothermic Reaction Lab Experiment.
From www.youtube.com
simple endothermic reaction YouTube Endothermic Reaction Lab Experiment Not all endothermic reactions are safe to touch, so their temperature change must be measured with a thermometer. Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). In this investigation, students classify chemical reactions. Endothermic Reaction Lab Experiment.
From www.youtube.com
CTSC practical experiment Endothermic & exothermic reactions YouTube Endothermic Reaction Lab Experiment Exothermic reactions may occur spontaneously and result in higher randomness or entropy (δs > 0) of the system. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Other chemical reactions release energy in the form of heat, light, or sound. This simple chemistry demonstration is an endothermic reaction. Endothermic Reaction Lab Experiment.
From www.feedback-shop.co.uk
The endothermic reaction Heat of reaction Energy in chemical Endothermic Reaction Lab Experiment This simple chemistry demonstration is an endothermic reaction that uses simple ingredients and produces a cooling effect that’s safe to touch. In this investigation, students classify chemical reactions as exothermic or endothermic. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Other chemical reactions release energy in the. Endothermic Reaction Lab Experiment.
From www.chemlabreport.com
Exothermic and Endothermic Reactions Experiments Lab Reports Endothermic Reaction Lab Experiment Endothermic reactions are characterized by positive heat flow (into the reaction) and an increase in enthalpy (+δh). Next, students explore the relationship between an observed change in temperature and the. Exothermic reactions could be harnessed to power machines or heat homes, while endothermic reactions could be used for treating injuries or. This simple chemistry demonstration is an endothermic reaction that. Endothermic Reaction Lab Experiment.