Safety Population Clinical Trial . The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals.
from mungfali.com
We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. The aims of this article are: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. (1) to describe a general framework for repeated monitoring of safety events in clinical trials;
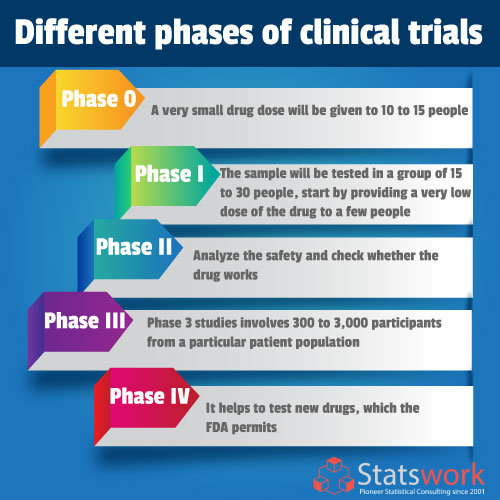
Phases Of Clinical Trials Chart
Safety Population Clinical Trial This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. An external validity analysis encompassing 43 895 trials and 5 685 738 individuals.
From www.template.net
Clinical Trial Safety Management Plan Template Edit Online & Download Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study.. Safety Population Clinical Trial.
From www.massbio.org
Balancing the Scale How Bristol Myers Squibb is Ensuring Clinical Safety Population Clinical Trial We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are: The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical. Safety Population Clinical Trial.
From www.researchgate.net
(PDF) Baricitinib Safety for Events of Special Interest in Populations Safety Population Clinical Trial This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. We share our recommendations for the statistical and. Safety Population Clinical Trial.
From crfweb.com
The Clincial Trial Process Stepbystep approach Safety Population Clinical Trial This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the. Safety Population Clinical Trial.
From www.jmp.com
RiskBased Monitoring in Clinical Trials Getting Started JMP Clinical Safety Population Clinical Trial The aims of this article are: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. This document addresses problems. Safety Population Clinical Trial.
From www.researchgate.net
Safety endpoints (pooled safety population) Download Scientific Diagram Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study.. Safety Population Clinical Trial.
From www.slideserve.com
PPT Safety Part I Clinical Trial Safety and Safety Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are (1) to. Safety Population Clinical Trial.
From lustgarten.org
Clinical Trial Phases Safety Population Clinical Trial We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are: This document addresses problems associated with clinical trials when there. Safety Population Clinical Trial.
From www.clinvigilant.com
3 different Ways Of Clinical Trial Monitoring ClinVigilant Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. An external validity analysis encompassing 43. Safety Population Clinical Trial.
From www.withpower.com
NTORM Emergency Preparedness for Vulnerable Populations Clinical Trial Safety Population Clinical Trial We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. The aims of this article are: (1) to describe a general framework for repeated monitoring of safety events in clinical trials; An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a. Safety Population Clinical Trial.
From www.researchgate.net
Primary safety endpoint results (intent to treat population) Download Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. This document addresses problems associated with clinical trials when there. Safety Population Clinical Trial.
From mungfali.com
Phases Of Clinical Trials Chart Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. This document addresses problems associated. Safety Population Clinical Trial.
From www.researchgate.net
Adverse Events during Treatment Safety Populations in Trials Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. We share our recommendations for the statistical and. Safety Population Clinical Trial.
From elearning.wfh.org
Monitoring Patient Safety in Clinical Trials eLearning Platform Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are: (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are (1) to. Safety Population Clinical Trial.
From www.slideserve.com
PPT Safety Part I Clinical Trial Safety and Safety Safety Population Clinical Trial This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the. Safety Population Clinical Trial.
From www.researchgate.net
in perprotocol and intentiontotreat populations Download Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are: The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; (1) to. Safety Population Clinical Trial.
From pharmdguru.com
16. SAFETY MONITORING IN CLINICAL TRIALS PHARMD GURU Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available. Safety Population Clinical Trial.
From www.researchgate.net
1 The phases of clinical trial research. Clinical trials pass through a Safety Population Clinical Trial The aims of this article are: This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to. Safety Population Clinical Trial.
From www.quantics.co.uk
What is a Safety Population in a Clinical Trial? Quantics Biostatistics Safety Population Clinical Trial The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated. Safety Population Clinical Trial.
From www.fshdsociety.org
What is a Clinical Trial? FSHD Society Safety Population Clinical Trial The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; An external validity analysis encompassing 43. Safety Population Clinical Trial.
From www.cloudbyz.com
How to Ensure Patient Safety During a Clinical Trial Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. This document. Safety Population Clinical Trial.
From www.researchgate.net
Flowchart of patient selection in GIDEON. a Safety population includes Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated. Safety Population Clinical Trial.
From www.researchgate.net
Summary of the clinical trials included in the total CCH safety Safety Population Clinical Trial The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. An external validity analysis encompassing. Safety Population Clinical Trial.
From evidence.nejm.org
Independent Oversight of Clinical Trials through Data and Safety Safety Population Clinical Trial The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are: (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. An external. Safety Population Clinical Trial.
From www.researchgate.net
Trial profile. The safety population was composed of all patients Safety Population Clinical Trial We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1). Safety Population Clinical Trial.
From within3.com
Demographic data in clinical trials Within3 Safety Population Clinical Trial The aims of this article are: We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a. Safety Population Clinical Trial.
From www.yumpu.com
safety reporting flowchart (PDF, 86.41 KB) Clinical Trials Toolkit Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; An external validity analysis encompassing 43. Safety Population Clinical Trial.
From www.researchgate.net
Trial profile The safety population consisted of patients who received Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated. Safety Population Clinical Trial.
From www.researchgate.net
Trial participant recruitment and randomization. The safety population Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary. Safety Population Clinical Trial.
From www.slideserve.com
PPT SAFETY MONITORING IN CLINICAL TRIALS PowerPoint Presentation ID Safety Population Clinical Trial The aims of this article are: The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report,. Safety Population Clinical Trial.
From nwflcrg.com
How Researchers Protect Patient Safety in Clinical Trials Safety Population Clinical Trial The aims of this article are: This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share. Safety Population Clinical Trial.
From health.gov.capital
How are vulnerable populations protected in clinical trials? Health Safety Population Clinical Trial This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. (1) to describe a general framework for repeated monitoring of safety events in. Safety Population Clinical Trial.
From pharmabeej.com
Patient Safety In Clinical Trials Pharmabeej Safety Population Clinical Trial This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. The aims of this article are: The aims of this article are (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report,. Safety Population Clinical Trial.
From thalassaemia.org.cy
Clinical Trials TIF Safety Population Clinical Trial (1) to describe a general framework for repeated monitoring of safety events in clinical trials; We share our recommendations for the statistical and graphical methodologies necessary to appropriately analyze, report, and. An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are (1) to describe a general framework for repeated monitoring of. Safety Population Clinical Trial.
From mrctcenter.org
clinical trial Clinical Research Glossary Safety Population Clinical Trial An external validity analysis encompassing 43 895 trials and 5 685 738 individuals. The aims of this article are: This document addresses problems associated with clinical trials when there are limited numbers of patients available to study. (1) to describe a general framework for repeated monitoring of safety events in clinical trials; The aims of this article are (1) to. Safety Population Clinical Trial.