Water Drawing Of Molecules . water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Determine the total number of valence electrons. water (h2o) should be drawn as two hydrogen atoms connected to one. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Move the entire molecule (you can already use the left. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. each tool has different behavior for the right mouse button: identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding.
from www.youtube.com
each tool has different behavior for the right mouse button: Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Move the entire molecule (you can already use the left. water (h2o) should be drawn as two hydrogen atoms connected to one. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Determine the total number of valence electrons.
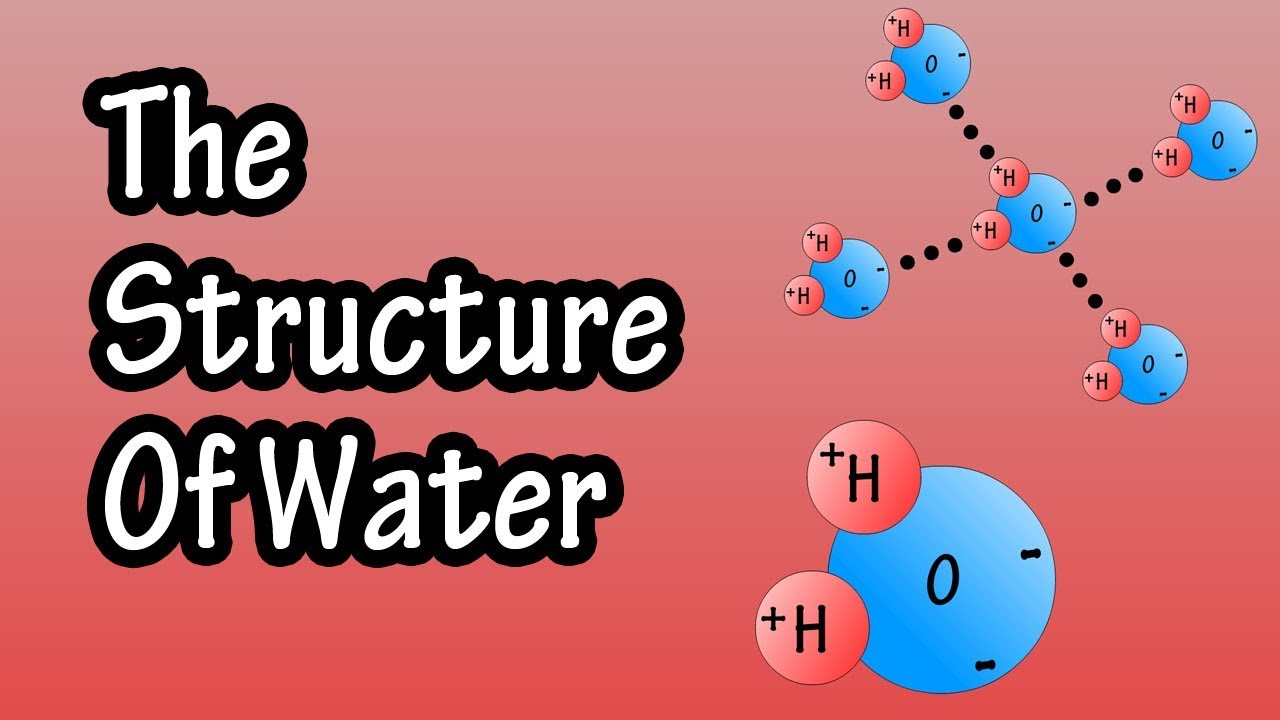
Structure Of Water Molecule Chemistry Of Water Properties Of Water
Water Drawing Of Molecules identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Determine the total number of valence electrons. each tool has different behavior for the right mouse button: water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Move the entire molecule (you can already use the left. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). water (h2o) should be drawn as two hydrogen atoms connected to one.
From aviewfromtheright.com
Water, Water Everywhere… Science, POLITICS, & Religion Water Drawing Of Molecules water (h2o) should be drawn as two hydrogen atoms connected to one. Move the entire molecule (you can already use the left. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Determine the total number of valence electrons. each tool has different behavior for the right mouse button: identify. Water Drawing Of Molecules.
From logyofbio.blogspot.com
bi·ol·o·gy (bīˈäləjē) Structure of a Water Molecule Water Drawing Of Molecules Determine the total number of valence electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. water is a simple molecule consisting of one oxygen. Water Drawing Of Molecules.
From mungfali.com
Polar Water Molecule Diagram Water Drawing Of Molecules Determine the total number of valence electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. identify three special properties of water that make it unusual for a molecule of its size, and explain. Water Drawing Of Molecules.
From www.vectorstock.com
H2o water molecule Royalty Free Vector Image VectorStock Water Drawing Of Molecules identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Move the entire molecule (you can already use the left. water (h2o) should be drawn as two hydrogen atoms connected to one. Because of the higher electronegativity of the oxygen atom, the bonds are. Water Drawing Of Molecules.
From kgrowth.blogspot.com
KGrowth What can we know about water molecule? Water Drawing Of Molecules Determine the total number of valence electrons. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. water (h2o) should be drawn as two hydrogen atoms connected to one. Explain what is meant by hydrogen bonding and the molecular structural features that bring it. Water Drawing Of Molecules.
From digitalpaxton.org
water molecule Water Drawing Of Molecules water (h2o) should be drawn as two hydrogen atoms connected to one. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Move the entire molecule (you can already use the left. Because of the higher electronegativity of the oxygen atom, the bonds are. Water Drawing Of Molecules.
From mavink.com
Water Molecules Hydrogen Bonding Water Drawing Of Molecules water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. water (h2o) should be drawn as two hydrogen. Water Drawing Of Molecules.
From www.alamy.com
Water molecule H2O. Vector illustration Stock Vector Image & Art Alamy Water Drawing Of Molecules Determine the total number of valence electrons. Move the entire molecule (you can already use the left. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. each. Water Drawing Of Molecules.
From www.britannica.com
water Definition, Chemical Formula, Structure, Molecule, & Facts Water Drawing Of Molecules Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. each tool has different behavior for the right mouse button: Determine the total number of valence. Water Drawing Of Molecules.
From pixels.com
Water Molecule Structure Photograph by Inna Bigun/science Photo Library Water Drawing Of Molecules Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Determine the total number of valence electrons. Move the entire molecule (you can already use the left. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. each tool has different behavior for the right mouse. Water Drawing Of Molecules.
From antasyaalinda.blogspot.com
Diagram Of Water Molecule Water Drawing Of Molecules Move the entire molecule (you can already use the left. each tool has different behavior for the right mouse button: Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of. Water Drawing Of Molecules.
From www.animalia-life.club
Water Molecule Structure For Kids Water Drawing Of Molecules Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Determine the total number of valence electrons. water (h2o) should be drawn as two hydrogen atoms connected to. Water Drawing Of Molecules.
From depositphotos.com
Water molecule sketch — Stock Vector © lhfgraphics 18320437 Water Drawing Of Molecules water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Move the entire molecule (you can already use the left. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. water (h2o) should be drawn as two. Water Drawing Of Molecules.
From www.vecteezy.com
Chemistry model of molecule water H2O scientific elements. Integrated Water Drawing Of Molecules Determine the total number of valence electrons. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Move the entire molecule (you can already use the left. Because of the higher electronegativity of the oxygen atom, the. Water Drawing Of Molecules.
From www.alamy.com
Water molecule Stock Photo Alamy Water Drawing Of Molecules each tool has different behavior for the right mouse button: Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). water is a simple molecule consisting of one oxygen atom bonded to two different. Water Drawing Of Molecules.
From www.dreamstime.com
Abstract Water Molecules Science and Chemistry Concept Stock Water Drawing Of Molecules Determine the total number of valence electrons. Move the entire molecule (you can already use the left. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. water (h2o) should be drawn as two hydrogen atoms connected to one. water is a simple molecule consisting of one oxygen atom bonded to two. Water Drawing Of Molecules.
From phys.org
Water molecules dance in three Water Drawing Of Molecules Move the entire molecule (you can already use the left. Determine the total number of valence electrons. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Explain. Water Drawing Of Molecules.
From www.e-education.psu.edu
The Configuration of the Water Molecule EARTH 111 Water Science and Water Drawing Of Molecules water (h2o) should be drawn as two hydrogen atoms connected to one. water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Move the entire molecule (you can already use the left. identify three special properties of water that make it unusual for a molecule of its size, and explain how. Water Drawing Of Molecules.
From fineartamerica.com
Water Molecule Digital Art by Laguna Design Fine Art America Water Drawing Of Molecules Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. each tool has different behavior for the right mouse button: Determine the total number of valence electrons. . Water Drawing Of Molecules.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure Water Drawing Of Molecules water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Determine the total number of valence electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). identify three special properties of water that make it unusual for a molecule of its size, and. Water Drawing Of Molecules.
From biostudizz.weebly.com
WATER CHEMISTRY to Bio Stud... Water Drawing Of Molecules water (h2o) should be drawn as two hydrogen atoms connected to one. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). each tool has. Water Drawing Of Molecules.
From hebasoffar.blogspot.com
Science online The importance of the water and its structure Water Drawing Of Molecules each tool has different behavior for the right mouse button: identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Move the entire molecule (you can. Water Drawing Of Molecules.
From cartoondealer.com
H2O Water Molecule Model And Chemical Formula Vector Illustration Water Drawing Of Molecules Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Move the entire molecule (you can already use the left. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Determine the total number of valence electrons.. Water Drawing Of Molecules.
From getrevising.co.uk
Physical & Chemical Properties of Water Revision Notes in University Water Drawing Of Molecules each tool has different behavior for the right mouse button: water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Move the entire molecule (you can already use the left. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). Explain what is meant. Water Drawing Of Molecules.
From study.com
Facts About Water Molecules Structure & Properties Video & Lesson Water Drawing Of Molecules Move the entire molecule (you can already use the left. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). each tool has different behavior for the right mouse button: Determine the total number of valence electrons. identify three special properties of water that make it unusual for a molecule. Water Drawing Of Molecules.
From taylorsciencegeeks.weebly.com
The Water Molecule Science News Water Drawing Of Molecules Move the entire molecule (you can already use the left. each tool has different behavior for the right mouse button: Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). identify three special properties of water that make it unusual for a molecule of its size, and explain how these. Water Drawing Of Molecules.
From www.pngitem.com
Picture Structural Diagram Of Water Molecule, HD Png Download Water Drawing Of Molecules Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Move the entire molecule (you can already use the left. Because of the higher electronegativity of the oxygen atom,. Water Drawing Of Molecules.
From www.vecteezy.com
Chemistry model of molecule water H2O scientific elements. Integrated Water Drawing Of Molecules Move the entire molecule (you can already use the left. Determine the total number of valence electrons. water (h2o) should be drawn as two hydrogen atoms connected to one. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. each tool has different. Water Drawing Of Molecules.
From openoregon.pressbooks.pub
Water Principles of Biology Water Drawing Of Molecules Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. water (h2o) should be drawn as two hydrogen atoms connected to one. Determine the total number of valence electrons. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). each tool has different behavior. Water Drawing Of Molecules.
From brainly.in
Draw a neat well labelled diagram of information of water molecule Water Drawing Of Molecules water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. water (h2o) should be drawn as two hydrogen atoms connected to one. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. each tool has different behavior for the right mouse button: Because of the. Water Drawing Of Molecules.
From jude-well-cowan.blogspot.com
Describe the Structure of a Water Molecule Water Drawing Of Molecules each tool has different behavior for the right mouse button: identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Determine the total number of valence electrons. Move the entire molecule (you can already use the left. Because of the higher electronegativity of the. Water Drawing Of Molecules.
From www.youtube.com
Structure Of Water Molecule Chemistry Of Water Properties Of Water Water Drawing Of Molecules each tool has different behavior for the right mouse button: Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Determine the total number of valence electrons. water (h2o) should be drawn as. Water Drawing Of Molecules.
From www.sciencephoto.com
Water molecule Stock Image A700/0381 Science Photo Library Water Drawing Of Molecules Determine the total number of valence electrons. each tool has different behavior for the right mouse button: Move the entire molecule (you can already use the left. water (h2o) should be drawn as two hydrogen atoms connected to one. Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. Because of the. Water Drawing Of Molecules.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. Water Drawing Of Molecules Move the entire molecule (you can already use the left. water (h2o) should be drawn as two hydrogen atoms connected to one. each tool has different behavior for the right mouse button: Explain what is meant by hydrogen bonding and the molecular structural features that bring it about. identify three special properties of water that make it. Water Drawing Of Molecules.
From getrevising.co.uk
Water and Carbohydrates Revision Cards in A Level and IB Biology Water Drawing Of Molecules water (h2o) should be drawn as two hydrogen atoms connected to one. Move the entire molecule (you can already use the left. Determine the total number of valence electrons. identify three special properties of water that make it unusual for a molecule of its size, and explain how these result from hydrogen bonding. Explain what is meant by. Water Drawing Of Molecules.