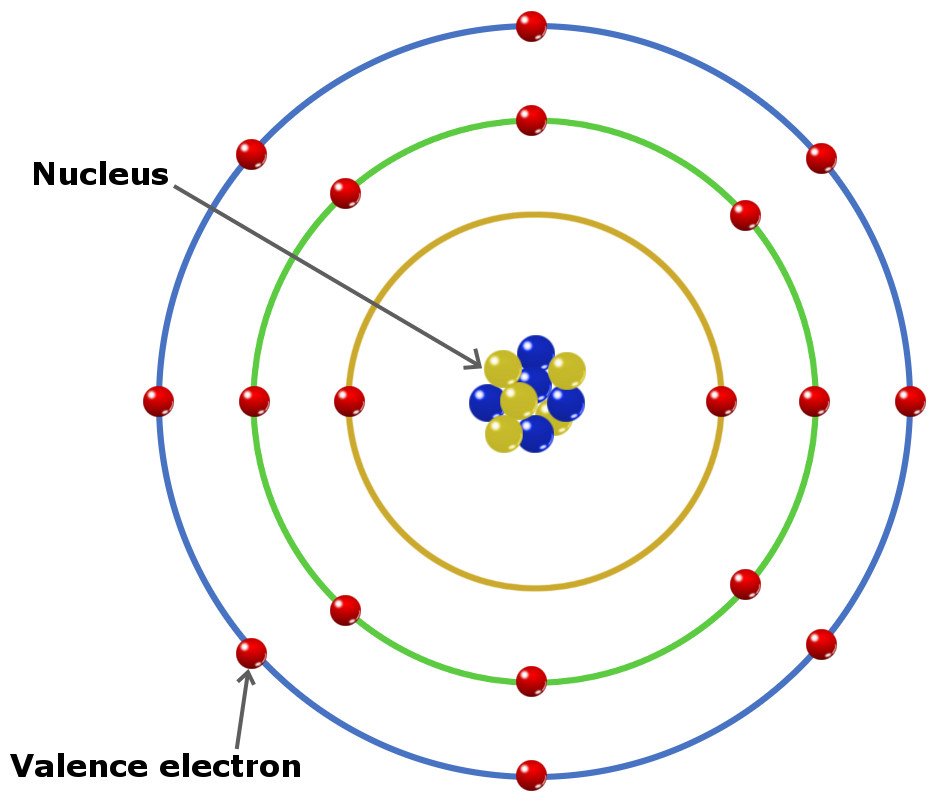
Do Atoms Have Electrons . A system of one or more electrons bound to a nucleus is called an atom. Part of chemistry (single science). The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. If the number of electrons is different from the nucleus's. Most atoms have three different subatomic particles inside them: The electronic structure of an atom can be predicted from its atomic number. Atoms contain protons, neutrons and electrons. All atoms are roughly the same size, whether they have 3 or 90 electrons. The electrons are arranged in shells around the nucleus. Approximately 50 million atoms of solid matter.
from www.scienceabc.com
The electronic structure of an atom can be predicted from its atomic number. The electrons are arranged in shells around the nucleus. Approximately 50 million atoms of solid matter. If the number of electrons is different from the nucleus's. All atoms are roughly the same size, whether they have 3 or 90 electrons. Atoms contain protons, neutrons and electrons. A system of one or more electrons bound to a nucleus is called an atom. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. Most atoms have three different subatomic particles inside them: Part of chemistry (single science).
What Are Valence Electrons and How To Find Them? Where Are They Located?
Do Atoms Have Electrons The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. All atoms are roughly the same size, whether they have 3 or 90 electrons. A system of one or more electrons bound to a nucleus is called an atom. Part of chemistry (single science). The electronic structure of an atom can be predicted from its atomic number. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. Approximately 50 million atoms of solid matter. Most atoms have three different subatomic particles inside them: If the number of electrons is different from the nucleus's. The electrons are arranged in shells around the nucleus. Atoms contain protons, neutrons and electrons.
From www.slideserve.com
PPT How many protons , electrons, and neutrons are in an atom Do Atoms Have Electrons Most atoms have three different subatomic particles inside them: Part of chemistry (single science). Atoms contain protons, neutrons and electrons. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. If the number of electrons is different from the. Do Atoms Have Electrons.
From www.expii.com
Valence Electrons — Definition & Importance Expii Do Atoms Have Electrons Atoms contain protons, neutrons and electrons. The electronic structure of an atom can be predicted from its atomic number. Approximately 50 million atoms of solid matter. Most atoms have three different subatomic particles inside them: The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very. Do Atoms Have Electrons.
From gzscienceclassonline.weebly.com
1. Electron Configuration Do Atoms Have Electrons If the number of electrons is different from the nucleus's. The electronic structure of an atom can be predicted from its atomic number. The electrons are arranged in shells around the nucleus. Part of chemistry (single science). Most atoms have three different subatomic particles inside them: Atoms contain protons, neutrons and electrons. All atoms are roughly the same size, whether. Do Atoms Have Electrons.
From www.howitworksdaily.com
Why do atoms have electrons? How It Works Do Atoms Have Electrons The electronic structure of an atom can be predicted from its atomic number. Atoms contain protons, neutrons and electrons. Most atoms have three different subatomic particles inside them: If the number of electrons is different from the nucleus's. A system of one or more electrons bound to a nucleus is called an atom. Part of chemistry (single science). All atoms. Do Atoms Have Electrons.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Do Atoms Have Electrons The electrons are arranged in shells around the nucleus. Part of chemistry (single science). Atoms contain protons, neutrons and electrons. Most atoms have three different subatomic particles inside them: Approximately 50 million atoms of solid matter. All atoms are roughly the same size, whether they have 3 or 90 electrons. A system of one or more electrons bound to a. Do Atoms Have Electrons.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica Do Atoms Have Electrons A system of one or more electrons bound to a nucleus is called an atom. Approximately 50 million atoms of solid matter. The electrons are arranged in shells around the nucleus. If the number of electrons is different from the nucleus's. Part of chemistry (single science). The protons and neutrons are packed together into the center of the atom (which. Do Atoms Have Electrons.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table Do Atoms Have Electrons Part of chemistry (single science). Most atoms have three different subatomic particles inside them: The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. All atoms are roughly the same size, whether they have 3 or 90 electrons. If. Do Atoms Have Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Do Atoms Have Electrons The electrons are arranged in shells around the nucleus. All atoms are roughly the same size, whether they have 3 or 90 electrons. Part of chemistry (single science). Atoms contain protons, neutrons and electrons. The electronic structure of an atom can be predicted from its atomic number. If the number of electrons is different from the nucleus's. Most atoms have. Do Atoms Have Electrons.
From courses.lumenlearning.com
Electrons Biology for Majors I Do Atoms Have Electrons The electronic structure of an atom can be predicted from its atomic number. All atoms are roughly the same size, whether they have 3 or 90 electrons. If the number of electrons is different from the nucleus's. Approximately 50 million atoms of solid matter. A system of one or more electrons bound to a nucleus is called an atom. Atoms. Do Atoms Have Electrons.
From www.scienceabc.com
Isotopes Definition, Explanation, Properties And Examples Do Atoms Have Electrons Part of chemistry (single science). A system of one or more electrons bound to a nucleus is called an atom. The electronic structure of an atom can be predicted from its atomic number. If the number of electrons is different from the nucleus's. All atoms are roughly the same size, whether they have 3 or 90 electrons. Atoms contain protons,. Do Atoms Have Electrons.
From kidspressmagazine.com
The Structure of Atoms Do Atoms Have Electrons Approximately 50 million atoms of solid matter. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. A system of one or more electrons bound to a nucleus is called an atom. All atoms are roughly the same size,. Do Atoms Have Electrons.
From www.electronicsandyou.com
Structure of an Atom Structure & Use of Electron & Proton in Electronics Do Atoms Have Electrons Atoms contain protons, neutrons and electrons. The electrons are arranged in shells around the nucleus. If the number of electrons is different from the nucleus's. Approximately 50 million atoms of solid matter. Most atoms have three different subatomic particles inside them: The protons and neutrons are packed together into the center of the atom (which is called the nucleus ). Do Atoms Have Electrons.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Do Atoms Have Electrons The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. All atoms are roughly the same size, whether they have 3 or 90 electrons. Atoms contain protons, neutrons and electrons. The electronic structure of an atom can be predicted. Do Atoms Have Electrons.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science Do Atoms Have Electrons The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. Most atoms have three different subatomic particles inside them: Part of chemistry (single science). Atoms contain protons, neutrons and electrons. All atoms are roughly the same size, whether they. Do Atoms Have Electrons.
From www.broadlearnings.com
Atomic Structure Broad Learnings Do Atoms Have Electrons The electrons are arranged in shells around the nucleus. The electronic structure of an atom can be predicted from its atomic number. Atoms contain protons, neutrons and electrons. All atoms are roughly the same size, whether they have 3 or 90 electrons. The protons and neutrons are packed together into the center of the atom (which is called the nucleus. Do Atoms Have Electrons.
From sites.google.com
Structure of the Atom Unit G Review Do Atoms Have Electrons Atoms contain protons, neutrons and electrons. All atoms are roughly the same size, whether they have 3 or 90 electrons. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. Most atoms have three different subatomic particles inside them:. Do Atoms Have Electrons.
From www.slideserve.com
PPT Summary of the Atom PowerPoint Presentation ID350838 Do Atoms Have Electrons The electronic structure of an atom can be predicted from its atomic number. Part of chemistry (single science). Approximately 50 million atoms of solid matter. The electrons are arranged in shells around the nucleus. Atoms contain protons, neutrons and electrons. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and. Do Atoms Have Electrons.
From www.vrogue.co
Why Do Atoms Have Electrons How It Works vrogue.co Do Atoms Have Electrons Most atoms have three different subatomic particles inside them: Part of chemistry (single science). Atoms contain protons, neutrons and electrons. If the number of electrons is different from the nucleus's. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the. Do Atoms Have Electrons.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do Atoms Have Electrons All atoms are roughly the same size, whether they have 3 or 90 electrons. Most atoms have three different subatomic particles inside them: The electronic structure of an atom can be predicted from its atomic number. The electrons are arranged in shells around the nucleus. Atoms contain protons, neutrons and electrons. The protons and neutrons are packed together into the. Do Atoms Have Electrons.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Do Atoms Have Electrons The electrons are arranged in shells around the nucleus. Approximately 50 million atoms of solid matter. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. The electronic structure of an atom can be predicted from its atomic number.. Do Atoms Have Electrons.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Do Atoms Have Electrons Approximately 50 million atoms of solid matter. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. The electrons are arranged in shells around the nucleus. Part of chemistry (single science). A system of one or more electrons bound. Do Atoms Have Electrons.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Do Atoms Have Electrons All atoms are roughly the same size, whether they have 3 or 90 electrons. Atoms contain protons, neutrons and electrons. Most atoms have three different subatomic particles inside them: Part of chemistry (single science). The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much. Do Atoms Have Electrons.
From sciencenotes.org
List of Electron Configurations of Elements Do Atoms Have Electrons A system of one or more electrons bound to a nucleus is called an atom. The electronic structure of an atom can be predicted from its atomic number. Part of chemistry (single science). Most atoms have three different subatomic particles inside them: If the number of electrons is different from the nucleus's. Approximately 50 million atoms of solid matter. Atoms. Do Atoms Have Electrons.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram Do Atoms Have Electrons A system of one or more electrons bound to a nucleus is called an atom. If the number of electrons is different from the nucleus's. The electronic structure of an atom can be predicted from its atomic number. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons,. Do Atoms Have Electrons.
From www.vrogue.co
Simple Model Of Atom Structure With Electrons Vector vrogue.co Do Atoms Have Electrons The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. Part of chemistry (single science). The electronic structure of an atom can be predicted from its atomic number. Most atoms have three different subatomic particles inside them: All atoms. Do Atoms Have Electrons.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Do Atoms Have Electrons A system of one or more electrons bound to a nucleus is called an atom. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. Most atoms have three different subatomic particles inside them: The electronic structure of an. Do Atoms Have Electrons.
From www.youtube.com
Atomic Structure And Electrons Structure Of An Atom What Are Atoms Do Atoms Have Electrons Most atoms have three different subatomic particles inside them: The electrons are arranged in shells around the nucleus. If the number of electrons is different from the nucleus's. Atoms contain protons, neutrons and electrons. The electronic structure of an atom can be predicted from its atomic number. Approximately 50 million atoms of solid matter. All atoms are roughly the same. Do Atoms Have Electrons.
From spmscience.blog.onlinetuition.com.my
4.2 Structure of Atoms SPM Science Do Atoms Have Electrons Most atoms have three different subatomic particles inside them: Approximately 50 million atoms of solid matter. The electrons are arranged in shells around the nucleus. A system of one or more electrons bound to a nucleus is called an atom. The electronic structure of an atom can be predicted from its atomic number. All atoms are roughly the same size,. Do Atoms Have Electrons.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art Do Atoms Have Electrons The electronic structure of an atom can be predicted from its atomic number. The electrons are arranged in shells around the nucleus. All atoms are roughly the same size, whether they have 3 or 90 electrons. Part of chemistry (single science). A system of one or more electrons bound to a nucleus is called an atom. Approximately 50 million atoms. Do Atoms Have Electrons.
From www.britannica.com
Atom Proton, Neutron, Nucleus Britannica Do Atoms Have Electrons The electrons are arranged in shells around the nucleus. All atoms are roughly the same size, whether they have 3 or 90 electrons. Most atoms have three different subatomic particles inside them: The electronic structure of an atom can be predicted from its atomic number. A system of one or more electrons bound to a nucleus is called an atom.. Do Atoms Have Electrons.
From www.animalia-life.club
Electrons In An Atom Do Atoms Have Electrons The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller, whizz around the outside. Part of chemistry (single science). Most atoms have three different subatomic particles inside them: If the number of electrons is different from the nucleus's. Atoms contain protons, neutrons and. Do Atoms Have Electrons.
From www.scienceabc.com
What Are Valence Electrons and How To Find Them? Where Are They Located? Do Atoms Have Electrons If the number of electrons is different from the nucleus's. Approximately 50 million atoms of solid matter. Most atoms have three different subatomic particles inside them: A system of one or more electrons bound to a nucleus is called an atom. The electronic structure of an atom can be predicted from its atomic number. Atoms contain protons, neutrons and electrons.. Do Atoms Have Electrons.
From solbergsphyscience.wikispaces.com
solbergsphyscience The Structure of the Atom Do Atoms Have Electrons Part of chemistry (single science). The electrons are arranged in shells around the nucleus. The electronic structure of an atom can be predicted from its atomic number. Atoms contain protons, neutrons and electrons. The protons and neutrons are packed together into the center of the atom (which is called the nucleus ) and the electrons, which are very much smaller,. Do Atoms Have Electrons.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Do Atoms Have Electrons If the number of electrons is different from the nucleus's. A system of one or more electrons bound to a nucleus is called an atom. Part of chemistry (single science). All atoms are roughly the same size, whether they have 3 or 90 electrons. Most atoms have three different subatomic particles inside them: The electrons are arranged in shells around. Do Atoms Have Electrons.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Do Atoms Have Electrons Atoms contain protons, neutrons and electrons. Part of chemistry (single science). Approximately 50 million atoms of solid matter. A system of one or more electrons bound to a nucleus is called an atom. All atoms are roughly the same size, whether they have 3 or 90 electrons. If the number of electrons is different from the nucleus's. The electrons are. Do Atoms Have Electrons.