Freeze-Drying Process Validation . Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to achieving successful validation of a freeze drying cycle:
from northslopechillers.com
This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,.
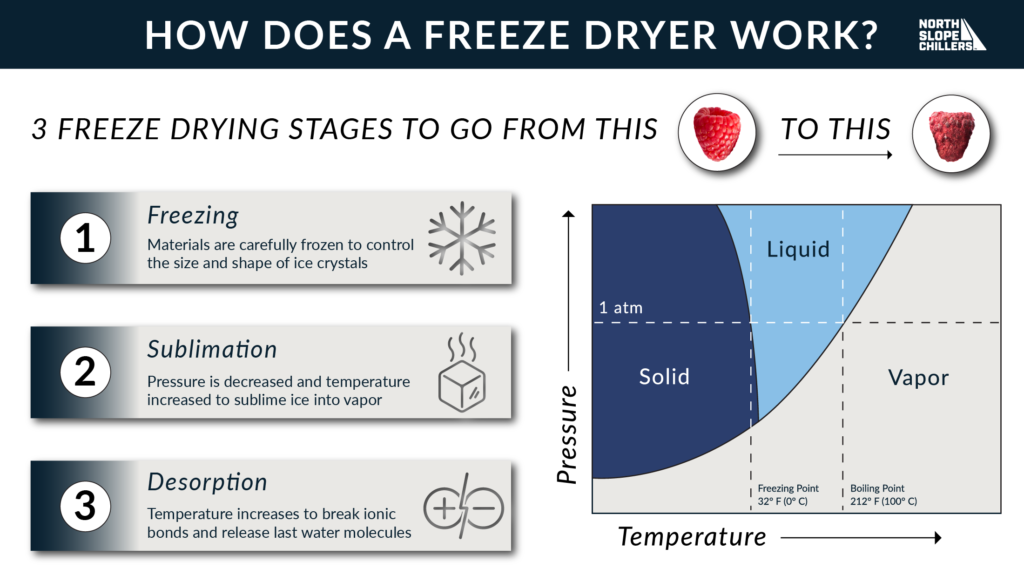
How Do Freeze Dryers Work? North Slope Chillers
Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require.
From www.semanticscholar.org
Process cycle development of freeze drying for therapeutic proteins Freeze-Drying Process Validation Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.pharmaceutical-technology.com
How to control the freeze drying process in demanding conditions Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From northslopechillers.com
How Do Freeze Dryers Work? North Slope Chillers Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.slideshare.net
Enhanced Process / Product Understanding and Control in Freeze Drying… Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.researchgate.net
(PDF) ModelBased Real Time Operation of the FreezeDrying Process Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to. Freeze-Drying Process Validation.
From jpharmsci.org
FreezeDrying of Pharmaceutical and Nutraceutical Nanoparticles The Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that. Freeze-Drying Process Validation.
From www.barnalab.com
Freezedrying preservation, everything you need to know Barnalab Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From barnalab.com
About freeze drying Freeze drying process Freeze-Drying Process Validation There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.researchgate.net
Schematic diagram of freeze drying. Download Scientific Diagram Freeze-Drying Process Validation There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From www.researchgate.net
Flow chart of freeze drying experiment for strawberry Download Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From teachntest.org
Freeze Dryer's Principle Construction Working Teachntest Pharma Freeze-Drying Process Validation Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Well defined and understood formulation, qualified freeze. There are three elements to achieving successful validation of a freeze drying cycle: This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From www.youtube.com
How Freeze Drying Works YouTube Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to. Freeze-Drying Process Validation.
From www.youtube.com
How to Operate FreezeDrying using State Diagram YouTube Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of. Freeze-Drying Process Validation.
From www.slideshare.net
Enhanced Process / Product Understanding and Control in Freeze Drying… Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.ellab.com
Freeze Drying Validation and Services Ellab Freeze-Drying Process Validation Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.youtube.com
Active Freeze Drying of pharmaceuticals YouTube Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are. Freeze-Drying Process Validation.
From www.ellab.com
Freeze Drying Validation and Services Ellab Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. There are three elements to. Freeze-Drying Process Validation.
From www.researchgate.net
Flow sheet of the freezedrying process Download Scientific Diagram Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From www.researchgate.net
PT diagram of freeze‐drying process Download Scientific Diagram Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to. Freeze-Drying Process Validation.
From www.slideshare.net
Enhanced Process / Product Understanding and Control in Freeze Drying… Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From www.mdpi.com
Processes Free FullText Spray FreezeDrying as a Solution to Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to. Freeze-Drying Process Validation.
From survival-mastery.com
How to Freeze Dry Food Instructions And Best Methods Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to achieving successful validation of a freeze drying cycle: This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Recommended best practices in monitoring of. Freeze-Drying Process Validation.
From healthcare-mittelhessen.eu
Freezedrying peptides in stable form Healthcare Mittelhessen Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to. Freeze-Drying Process Validation.
From www.researchgate.net
2. Schematic representation of the overall freezedrying process Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.barnalab.com
About freeze drying Freeze drying process Freeze-Drying Process Validation Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to achieving successful validation of a freeze drying. Freeze-Drying Process Validation.
From jpharmsci.org
The Importance of Understanding the Freezing Step and Its Impact on Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. There are three elements to achieving successful validation of a freeze drying cycle: This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Recommended best practices in monitoring of. Freeze-Drying Process Validation.
From www.horiba.com
Vacuum Monitoring in Dryer Equipment for Freeze Dry in Pharmaceuticals Freeze-Drying Process Validation Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. There are three elements to. Freeze-Drying Process Validation.
From vekuma.com
Freeze Drying Process Vekuma Freeze Dryers Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Recommended best practices in monitoring of. Freeze-Drying Process Validation.
From www.researchgate.net
The flow chart of the freeze‐drying system Download Scientific Diagram Freeze-Drying Process Validation Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. There are three elements to. Freeze-Drying Process Validation.
From hosokawa.com.my
How Active Freeze Drying simplifies validation for pharmaceutical Freeze-Drying Process Validation Well defined and understood formulation, qualified freeze. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.ellab.com
Freeze Drying Validation and Services Ellab Freeze-Drying Process Validation Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.
From www.medicago.se
Freeze Drying Medicago Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Recommended best practices in monitoring of. Freeze-Drying Process Validation.
From mungfali.com
Freeze Drying Process Flow Chart Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. There are three elements to achieving successful validation of a freeze drying cycle: Well defined and understood formulation, qualified freeze. Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From www.provac.com
The Process of Freeze Drying Under Vacuum Freeze-Drying Process Validation This document provides an overview of freeze drying and considerations for establishing the cycle validation strategy for the lyophilization. Well defined and understood formulation, qualified freeze. There are three elements to achieving successful validation of a freeze drying cycle: Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. Freeze drying, or lyophilization, has become an. Freeze-Drying Process Validation.
From vikumer.com
Freeze Drying Theory Vikumer Freeze Dry Freeze-Drying Process Validation Recommended best practices in monitoring of product status during pharmaceutical freeze drying are presented,. There are three elements to achieving successful validation of a freeze drying cycle: Freeze drying, or lyophilization, has become an accepted method of processing sensitive products that require. Well defined and understood formulation, qualified freeze. This document provides an overview of freeze drying and considerations for. Freeze-Drying Process Validation.