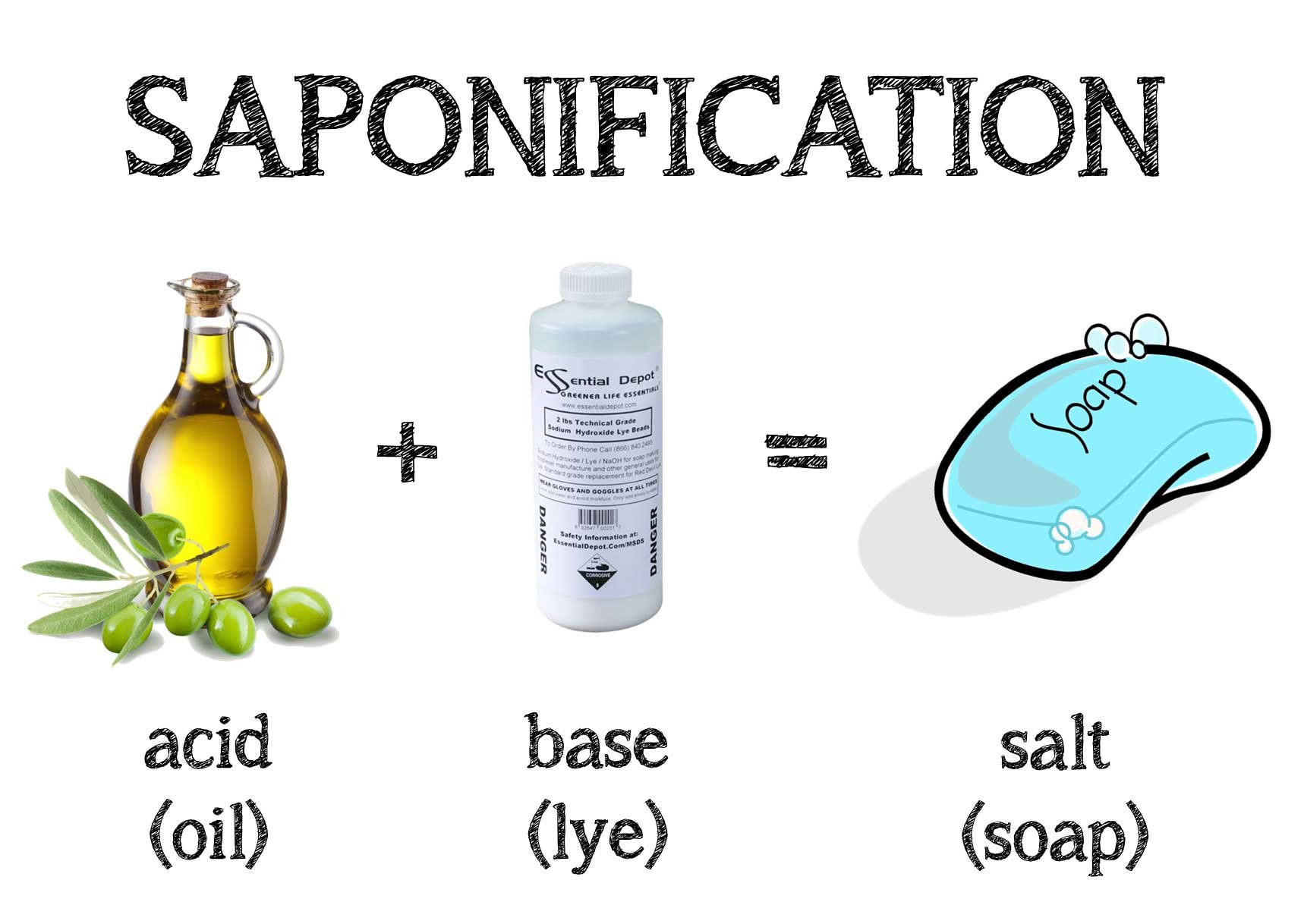
How Is Soap Made Chemically . Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Each soap molecule has a long. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Natural soaps are sodium or potassium salts of. This combination creates clusters of soap, water, and grime called micelles. Their molecules must contain a hydrophobic (water. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification.
from busy.org
One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Their molecules must contain a hydrophobic (water. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Natural soaps are sodium or potassium salts of. This combination creates clusters of soap, water, and grime called micelles. Each soap molecule has a long.
Saponification 1/2
How Is Soap Made Chemically One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Each soap molecule has a long. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Natural soaps are sodium or potassium salts of. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Their molecules must contain a hydrophobic (water. This combination creates clusters of soap, water, and grime called micelles.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis Describe the uses of organic How Is Soap Made Chemically Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. This combination creates clusters of soap, water, and grime called micelles. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or. How Is Soap Made Chemically.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 How Is Soap Made Chemically Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap. How Is Soap Made Chemically.
From www.pinterest.ph
Hand washing with soap vector illustration. Educational explanation scheme. Labeled process How Is Soap Made Chemically Each soap molecule has a long. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and. How Is Soap Made Chemically.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID6534085 How Is Soap Made Chemically Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long. Natural soaps are sodium or potassium salts of. One of the organic chemical reactions. How Is Soap Made Chemically.
From www.sharpestarena.com
Production of Soap Complete Project on Soap Making How Is Soap Made Chemically Natural soaps are sodium or potassium salts of. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Each soap. How Is Soap Made Chemically.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more How Is Soap Made Chemically Their molecules must contain a hydrophobic (water. This combination creates clusters of soap, water, and grime called micelles. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to. How Is Soap Made Chemically.
From busy.org
Saponification 1/2 How Is Soap Made Chemically Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long. This combination creates clusters of soap, water, and grime called micelles. One of the. How Is Soap Made Chemically.
From www.slideserve.com
PPT How is Soap Made? PowerPoint Presentation, free download ID2530430 How Is Soap Made Chemically This combination creates clusters of soap, water, and grime called micelles. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or. How Is Soap Made Chemically.
From www.youtube.com
Chemistry 101 How does soap work? YouTube How Is Soap Made Chemically How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Natural soaps are sodium or potassium salts of. One of. How Is Soap Made Chemically.
From www.thoughtco.com
How Soap Works How Is Soap Made Chemically One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap. How Is Soap Made Chemically.
From www.pinterest.com
Chemical Reactions Chemical reactions, Soap, Soap making How Is Soap Made Chemically Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. This combination creates clusters of soap, water, and grime called micelles. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. How soap works is due to its unique chemistry,. How Is Soap Made Chemically.
From lilis-chemistryblog.weebly.com
Soap Making Chemistry Blog How Is Soap Made Chemically How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. One of the organic chemical reactions known to ancient man. How Is Soap Made Chemically.
From www.soaphistory.net
How Soap is Made and Soap Ingredients How Is Soap Made Chemically Each soap molecule has a long. This combination creates clusters of soap, water, and grime called micelles. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine. How Is Soap Made Chemically.
From lilis-chemistryblog.weebly.com
Soap Making Chemistry Blog How Is Soap Made Chemically Natural soaps are sodium or potassium salts of. Each soap molecule has a long. This combination creates clusters of soap, water, and grime called micelles. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. One of the organic chemical. How Is Soap Made Chemically.
From www.reagent.co.uk
The Chemistry Of Soap Making The Science Blog How Is Soap Made Chemically Their molecules must contain a hydrophobic (water. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Natural soaps are sodium or potassium salts of. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water. How Is Soap Made Chemically.
From www.fix.com
Soap Making Basics How Is Soap Made Chemically Natural soaps are sodium or potassium salts of. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. This combination creates clusters of soap, water, and grime called micelles. How. How Is Soap Made Chemically.
From www.slideserve.com
PPT How is Soap Made? PowerPoint Presentation, free download ID2530430 How Is Soap Made Chemically Each soap molecule has a long. Natural soaps are sodium or potassium salts of. Their molecules must contain a hydrophobic (water. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called. How Is Soap Made Chemically.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 How Is Soap Made Chemically One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. This combination creates clusters of soap, water, and grime called micelles. Natural soaps are sodium or potassium salts of. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Soaps. How Is Soap Made Chemically.
From www.reddit.com
A cool guide about the Basic steps of soap making r/coolguides How Is Soap Made Chemically One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Their molecules must contain a hydrophobic (water. Soap is made from reacting a fat or oil (or a mixture) with. How Is Soap Made Chemically.
From brainly.in
what is the process to make soap ? Brainly.in How Is Soap Made Chemically Their molecules must contain a hydrophobic (water. Each soap molecule has a long. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. This combination creates clusters of soap, water, and grime called micelles. Soap is made from reacting a fat or oil (or a mixture) with a strong base. How Is Soap Made Chemically.
From www.thesprucecrafts.com
Learn How to Make Homemade Soap How Is Soap Made Chemically This combination creates clusters of soap, water, and grime called micelles. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called. How Is Soap Made Chemically.
From www.pinterest.com
How Is Soap Made? Learn the Science and the Art of Saponification. (With images) Soap making How Is Soap Made Chemically This combination creates clusters of soap, water, and grime called micelles. Natural soaps are sodium or potassium salts of. Each soap molecule has a long. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Their molecules must contain a hydrophobic (water. One of the organic chemical reactions known to. How Is Soap Made Chemically.
From www.youtube.com
How does Soap Work? YouTube How Is Soap Made Chemically Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. This combination creates clusters of soap, water, and grime called micelles. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or. How Is Soap Made Chemically.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents // HSC Chemistry YouTube How Is Soap Made Chemically Each soap molecule has a long. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Natural soaps are sodium or potassium salts of. This combination creates clusters of soap, water, and grime called micelles. Soaps are sodium or potassium. How Is Soap Made Chemically.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis Describe the uses of organic How Is Soap Made Chemically How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. One of the organic chemical reactions known to ancient man. How Is Soap Made Chemically.
From www.slideshare.net
Chemistry of soaps How Is Soap Made Chemically Natural soaps are sodium or potassium salts of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Their molecules must contain a hydrophobic (water. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Each soap molecule has a. How Is Soap Made Chemically.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Muffin Beauty Science How Is Soap Made Chemically Their molecules must contain a hydrophobic (water. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Natural soaps are sodium or potassium salts of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. This combination creates clusters of. How Is Soap Made Chemically.
From my-chem-assignment.blogspot.com
Chemistry Assignment How Is Soap Made Chemically Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Soaps are sodium or potassium fatty acids salts, produced from. How Is Soap Made Chemically.
From www.cosycottagesoap.co.uk
The Chemistry of Soap Making Cosy Cottage Soap How Is Soap Made Chemically Each soap molecule has a long. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. One of the organic chemical reactions known to ancient man was the preparation of. How Is Soap Made Chemically.
From www.fix.com
Soap Making Basics How Is Soap Made Chemically Each soap molecule has a long. Their molecules must contain a hydrophobic (water. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. This combination creates clusters of soap, water, and grime called micelles. Natural soaps are sodium or potassium salts of. One of the organic chemical reactions known to. How Is Soap Made Chemically.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences How Is Soap Made Chemically One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Their molecules must contain a hydrophobic (water. This combination creates. How Is Soap Made Chemically.
From cen.acs.org
Periodic graphics Soap versus body wash How Is Soap Made Chemically Their molecules must contain a hydrophobic (water. This combination creates clusters of soap, water, and grime called micelles. Each soap molecule has a long. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a. How Is Soap Made Chemically.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry How Is Soap Made Chemically Their molecules must contain a hydrophobic (water. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Natural soaps are sodium or potassium salts of. Soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in. How Is Soap Made Chemically.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID6534085 How Is Soap Made Chemically Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. How soap works is due to its unique chemistry, the hydrophilic (loves water) and hydrophobic (hates water) parts of soap act to combine soapy water with grease, dirt, or oil. Their molecules must contain a hydrophobic (water. One of the. How Is Soap Made Chemically.
From www.susansoaps.com
How is Soap Made? How Is Soap Made Chemically Natural soaps are sodium or potassium salts of. Each soap molecule has a long. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high. This combination creates clusters of soap, water, and grime called micelles. Their molecules must contain a hydrophobic (water. Soaps are sodium or potassium fatty acids salts,. How Is Soap Made Chemically.