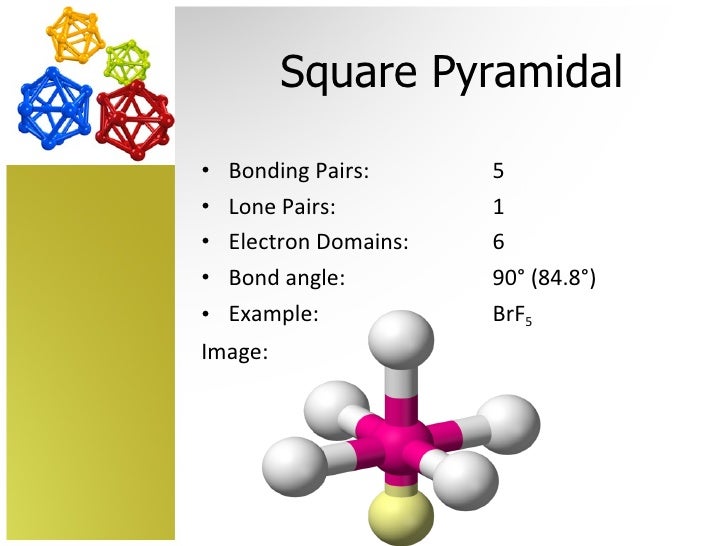
Pyramidal Molecule Definition . Molecular geometry is tetrahedral (e.g. This molecule shape resembles a pyramid with a triangular base. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing.
from www.slideshare.net
With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. Molecular geometry is tetrahedral (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. This molecule shape resembles a pyramid with a triangular base. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure.
Molecular Geometry
Pyramidal Molecule Definition When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. Molecular geometry is tetrahedral (e.g. This molecule shape resembles a pyramid with a triangular base. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing.
From ar.inspiredpencil.com
Trigonal Pyramidal Examples Pyramidal Molecule Definition With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. This molecule shape resembles a pyramid with a triangular base. Molecular geometry is tetrahedral (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single. Pyramidal Molecule Definition.
From www.slideserve.com
PPT Drawing Lewis structures PowerPoint Presentation, free download Pyramidal Molecule Definition This molecule shape resembles a pyramid with a triangular base. Molecular geometry is tetrahedral (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. When the central atom in a molecule has. Pyramidal Molecule Definition.
From www.slideserve.com
PPT Chapter 10 Chemical Bonding II PowerPoint Presentation, free Pyramidal Molecule Definition (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. Molecular geometry is tetrahedral (e.g. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal. Pyramidal Molecule Definition.
From www.slideserve.com
PPT Chapter 10 Chemical Bonding II PowerPoint Presentation, free Pyramidal Molecule Definition This molecule shape resembles a pyramid with a triangular base. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing. Pyramidal Molecule Definition.
From www.dreamstime.com
Molecular Geometry Structure of Elements Stock Vector Illustration of Pyramidal Molecule Definition When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. This molecule shape resembles a pyramid with a triangular base. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing. Pyramidal Molecule Definition.
From www.webassign.net
Chapter 6 Molecular Structure Pyramidal Molecule Definition Molecular geometry is tetrahedral (e.g. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. This molecule shape resembles a pyramid with a triangular base. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. (c) the actual bond angles deviate. Pyramidal Molecule Definition.
From www.vhv.rs
File Ax5e02d Square Pyramidal Molecular Geometry, HD Png Download Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. This molecule shape resembles a pyramid with a triangular base. When the central atom in a molecule has three bonds and. Pyramidal Molecule Definition.
From en.academic.ru
Trigonal pyramidal molecular geometry Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. Molecular geometry is tetrahedral (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. This molecule shape resembles a pyramid with a triangular base. (c) the actual. Pyramidal Molecule Definition.
From higher-secondary-chemistry.blogspot.com
Higher Secondary Chemistry Chapter 4.20 Square Pyramidal Shape Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex. Pyramidal Molecule Definition.
From favpng.com
Ammonia Trigonal Pyramidal Molecular Geometry Molecule Lone Pair, PNG Pyramidal Molecule Definition Molecular geometry is tetrahedral (e.g. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. (c) the actual bond angles deviate slightly from the idealized angles because the. Pyramidal Molecule Definition.
From www.youtube.com
Square Pyramidal Molecular Geometry/Shape and Bond Angles YouTube Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. Molecular geometry is tetrahedral (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. This molecule shape resembles a pyramid with a triangular base. (c) the actual. Pyramidal Molecule Definition.
From www.istockphoto.com
3d Illustration Of A Molecular Model Of A Triangular Pyramid With A Pyramidal Molecule Definition With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. This molecule shape resembles. Pyramidal Molecule Definition.
From mungfali.com
Trigonal Pyramidal Structure Pyramidal Molecule Definition (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. Molecular geometry is tetrahedral (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on. Pyramidal Molecule Definition.
From chem.libretexts.org
6.E Lewis Acids and Molecular Shapes (Exercises) Chemistry LibreTexts Pyramidal Molecule Definition This molecule shape resembles a pyramid with a triangular base. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. With five nuclei surrounding the central atom, the. Pyramidal Molecule Definition.
From sketchfab.com
Square Pyramidal Molecular Geometry Download Free 3D model by orgoly Pyramidal Molecule Definition This molecule shape resembles a pyramid with a triangular base. Molecular geometry is tetrahedral (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. With five nuclei surrounding the central atom, the. Pyramidal Molecule Definition.
From www.researchgate.net
of the pyramidal molecules studied in this work and the factors thought Pyramidal Molecule Definition When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. This molecule shape resembles a pyramid with a triangular base. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing. Pyramidal Molecule Definition.
From www.slideserve.com
PPT Shapes of Molecules PowerPoint Presentation, free download ID Pyramidal Molecule Definition When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex. Pyramidal Molecule Definition.
From www.expii.com
Ideal Bond Angles — Overview & Examples Expii Pyramidal Molecule Definition Molecular geometry is tetrahedral (e.g. This molecule shape resembles a pyramid with a triangular base. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the. Pyramidal Molecule Definition.
From courses.lumenlearning.com
Molecular Structure and Polarity CHEM 1305 Introductory Chemistry Pyramidal Molecule Definition With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. Molecular geometry is tetrahedral (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than. Pyramidal Molecule Definition.
From animalia-life.club
Trigonal Pyramidal Bond Angle Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. When the central. Pyramidal Molecule Definition.
From cartridges.esciencelabs.com
Diagram of shapes of molecules, showng bonding pairs, arrangement of Pyramidal Molecule Definition Molecular geometry is tetrahedral (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. With five nuclei surrounding the central atom, the molecular structure is based on. Pyramidal Molecule Definition.
From mungfali.com
Trigonal Pyramidal Lewis Dot Structure Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. This molecule shape resembles a pyramid with a triangular base. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. With five nuclei surrounding the central atom, the. Pyramidal Molecule Definition.
From byjus.com
Name a molecule that has a trigonal pyramidal shape. Pyramidal Molecule Definition (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. With. Pyramidal Molecule Definition.
From chem.libretexts.org
9.7 The Shapes of Molecules Chemistry LibreTexts Pyramidal Molecule Definition With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. Molecular geometry is tetrahedral (e.g. If there is one lone pair of electrons and three bond pairs the resulting. Pyramidal Molecule Definition.
From www.bqua.com
Trigonal Pyramidal Molecular Geometry Archives BQUA Pyramidal Molecule Definition When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. This molecule shape resembles a pyramid with a triangular base. Molecular geometry is tetrahedral (e.g. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. (c) the actual. Pyramidal Molecule Definition.
From ar.inspiredpencil.com
Square Pyramidal Molecular Geometry Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a. Pyramidal Molecule Definition.
From www.youtube.com
Trigonal Pyramidal Molecular Geometry/Shape and Bond Angles YouTube Pyramidal Molecule Definition With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. This molecule shape resembles. Pyramidal Molecule Definition.
From oneclass.com
CHEM 1201 Lecture Notes Summer 2014, Lecture 9 Electronegativity Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal. Pyramidal Molecule Definition.
From alchetron.com
Square pyramidal molecular geometry Alchetron, the free social Pyramidal Molecule Definition With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle to be slightly smaller than 109.5°. When the central atom. Pyramidal Molecule Definition.
From study.com
VSEPR Theory Chart & Model Lesson Pyramidal Molecule Definition When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. Molecular geometry is tetrahedral (e.g. This molecule shape resembles a pyramid with a triangular base. (c) the actual. Pyramidal Molecule Definition.
From www.wikiwand.com
Géométrie moléculaire pyramidale trigonale Wikiwand Pyramidal Molecule Definition Molecular geometry is tetrahedral (e.g. This molecule shape resembles a pyramid with a triangular base. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than. Pyramidal Molecule Definition.
From www.siyavula.com
3.2 Molecular shape Atomic combinations Siyavula Pyramidal Molecule Definition This molecule shape resembles a pyramid with a triangular base. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single bonds, causing the hnh angle. Pyramidal Molecule Definition.
From www.slideshare.net
Molecular Geometry Pyramidal Molecule Definition With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. Molecular geometry is tetrahedral (e.g. This molecule shape resembles a pyramid with a triangular base. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a larger region of space than do the single. Pyramidal Molecule Definition.
From www.masterorganicchemistry.com
What Are Hybrid Orbitals? Master Organic Chemistry Pyramidal Molecule Definition Molecular geometry is tetrahedral (e.g. With five nuclei surrounding the central atom, the molecular structure is based on an octahedron with a vertex missing. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. This molecule shape resembles a pyramid with a triangular base. If there is one. Pyramidal Molecule Definition.
From higher-secondary-chemistry.blogspot.com
Higher Secondary Chemistry Chapter 4.20 Square Pyramidal Shape Pyramidal Molecule Definition If there is one lone pair of electrons and three bond pairs the resulting molecular geometry is trigonal pyramidal (e.g. When the central atom in a molecule has three bonds and one lone pair, the molecule takes on a trigonal pyramidal structure. (c) the actual bond angles deviate slightly from the idealized angles because the lone pair takes up a. Pyramidal Molecule Definition.