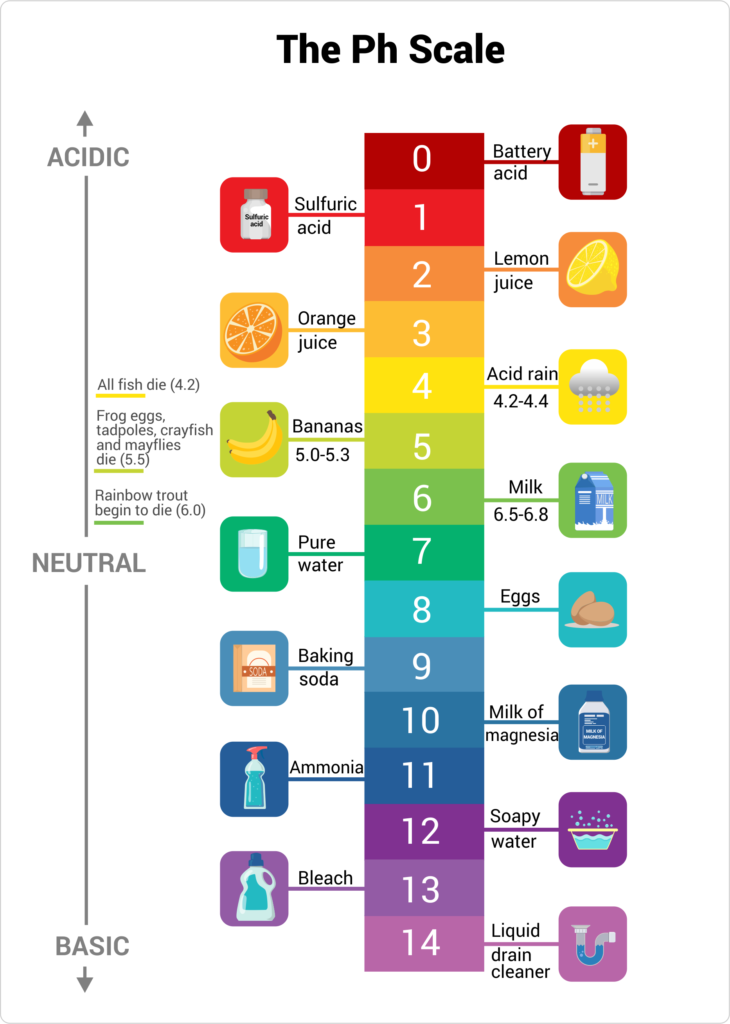
Vinegar Ph Vs Concentration . Two types of vinegar with equal levels of acidity can also have different ph values. However, adding water to vinegar can never turn vinegar into an. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Two varieties of vinegar with the same level of acidity can have different ph values. Ph is the measure of hydrogen ion. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. On a technical level, vinegar is a solution of acetic. Vinegar is acidic because of its low ph.
from www.mometrix.com
On a technical level, vinegar is a solution of acetic. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Vinegar is acidic because of its low ph. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Two varieties of vinegar with the same level of acidity can have different ph values. Two types of vinegar with equal levels of acidity can also have different ph values. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Ph is the measure of hydrogen ion. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition.
pH Overview (Chemistry Review Video)
Vinegar Ph Vs Concentration On a technical level, vinegar is a solution of acetic. Two varieties of vinegar with the same level of acidity can have different ph values. On a technical level, vinegar is a solution of acetic. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Two types of vinegar with equal levels of acidity can also have different ph values. Ph is the measure of hydrogen ion. However, adding water to vinegar can never turn vinegar into an. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Vinegar is acidic because of its low ph. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition.
From fphoto.photoshelter.com
science chemistry acid base ph meter Fundamental Photographs The Vinegar Ph Vs Concentration Two types of vinegar with equal levels of acidity can also have different ph values. Two varieties of vinegar with the same level of acidity can have different ph values. However, adding water to vinegar can never turn vinegar into an. Vinegar is acidic because of its low ph. On a technical level, vinegar is a solution of acetic. Most. Vinegar Ph Vs Concentration.
From calculatorshub.net
Vinegar Dilution pH Calculator Online Vinegar Ph Vs Concentration However, adding water to vinegar can never turn vinegar into an. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. On a technical level, vinegar is a solution of acetic. Two varieties of. Vinegar Ph Vs Concentration.
From blog.kanelstrand.com
Kanelstrand Which is Better for Hair Lemon Juice or Vinegar? Vinegar Ph Vs Concentration Vinegar is acidic because of its low ph. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. However, adding water to vinegar can never turn vinegar into an. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Two varieties of vinegar with. Vinegar Ph Vs Concentration.
From www.toppr.com
Question 41. The pH of a sample of vinegar is 3.76. Calculate the Vinegar Ph Vs Concentration Two varieties of vinegar with the same level of acidity can have different ph values. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Vinegar is acidic because of its low ph. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Two types of. Vinegar Ph Vs Concentration.
From ifocushealth.com
Will Apple Cider Vinegar Really Make You Healthy? Vinegar Ph Vs Concentration Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Two varieties of vinegar with the same level of acidity can have different ph values. Ph is the measure of the. Vinegar Ph Vs Concentration.
From realgoodgummies.com
Apple Cider Vinegar Vs. White Vinegar Know The Difference Vinegar Ph Vs Concentration Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Two types of vinegar with equal levels of acidity can also have different ph values. On a technical level, vinegar is a solution of acetic. Vinegar is acidic because of its low ph. Acetic acid is what distinguishes vinegar and. Vinegar Ph Vs Concentration.
From www.pinterest.com
vinegar acidity chart Google Search Chemistry Basics, Chemistry Notes Vinegar Ph Vs Concentration Two types of vinegar with equal levels of acidity can also have different ph values. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Two varieties of vinegar with the same level of acidity can have different ph values. Diluting vinegar with water increases its ph value, because vinegar is an acid. Vinegar Ph Vs Concentration.
From byjus.com
pH Of Acids And Bases Calculate pH Value Chemistry Byju's Vinegar Ph Vs Concentration Two varieties of vinegar with the same level of acidity can have different ph values. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. However, adding water to vinegar can never turn. Vinegar Ph Vs Concentration.
From www.youtube.com
The pH of a sample of vinegar is 3.76. Calculate the concentration of Vinegar Ph Vs Concentration Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. On a technical level, vinegar is a solution of acetic. Ph is the measure of hydrogen ion. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Vinegar is acidic because of its low. Vinegar Ph Vs Concentration.
From www.youtube.com
How to test the pH of Vinegar PH Test For Vinegar YouTube Vinegar Ph Vs Concentration Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Two types of vinegar with equal levels of acidity can also have different ph values. Ph is the measure of the hydrogen ion. Vinegar Ph Vs Concentration.
From postusing.blogspot.com
White Vinegar Ph Level / Ph And Acidity Their Difference And Importance Vinegar Ph Vs Concentration However, adding water to vinegar can never turn vinegar into an. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. On a technical level, vinegar is a solution of acetic. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Consuming it doesn’t. Vinegar Ph Vs Concentration.
From theapplecidervinegar.com
we have found What is the ph of apple cider vinegar Apple Cider Vinegar Vinegar Ph Vs Concentration Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. On a technical level, vinegar is a solution of acetic. However, adding water to vinegar can never turn vinegar into an. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Two varieties. Vinegar Ph Vs Concentration.
From www.researchgate.net
Variation of concentration and pH of samples Download Table Vinegar Ph Vs Concentration Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. However, adding water to vinegar can never turn vinegar into an. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Consuming it doesn’t affect your body’s natural ph levels, which. Vinegar Ph Vs Concentration.
From www.researchgate.net
Calculated pH using OLI software for HCl solutions as a function of Vinegar Ph Vs Concentration Vinegar is acidic because of its low ph. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. However, adding water to vinegar can never turn vinegar into an. Two types. Vinegar Ph Vs Concentration.
From www.youtube.com
Apple cider vinegar ph YouTube Vinegar Ph Vs Concentration Vinegar is acidic because of its low ph. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple. Vinegar Ph Vs Concentration.
From konsumenlistrik.com
What is the pH of Vinegar? Vinegar Ph Vs Concentration Two types of vinegar with equal levels of acidity can also have different ph values. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. However, adding water to vinegar can. Vinegar Ph Vs Concentration.
From study.com
Acidic, Basic & Neutral Solutions Overview, pH Scale & Uses Lesson Vinegar Ph Vs Concentration Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Two types of vinegar with equal levels of acidity can also have different ph values. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Two varieties of vinegar with the same level of. Vinegar Ph Vs Concentration.
From www.youtube.com
pH of Vinegar Is vinegar an acid or base? YouTube Vinegar Ph Vs Concentration Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. However, adding water to vinegar can never turn vinegar into an. Vinegar is acidic because of its low ph. On a technical level, vinegar is a solution of acetic. Consuming it doesn’t affect your body’s natural ph levels, which stay stable. Vinegar Ph Vs Concentration.
From www.chegg.com
Solved Determination of Acetic Acid Concentration in Vinegar Vinegar Ph Vs Concentration Two varieties of vinegar with the same level of acidity can have different ph values. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Two types of vinegar with equal levels of acidity can also have different ph values. On a technical level, vinegar is a solution of acetic. Consuming it doesn’t. Vinegar Ph Vs Concentration.
From www.wonderopolis.org
What Happens When You Mix Vinegar and Baking Soda? Wonderopolis Vinegar Ph Vs Concentration Ph is the measure of hydrogen ion. However, adding water to vinegar can never turn vinegar into an. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Two varieties of vinegar with the. Vinegar Ph Vs Concentration.
From greengobbler.com
What is the pH of Vinegar? Green Gobbler Vinegar Ph Vs Concentration However, adding water to vinegar can never turn vinegar into an. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. On a technical level, vinegar is a solution of acetic. Vinegar is acidic because of its low ph. Ph is the measure of hydrogen ion. Acetic acid is what distinguishes vinegar and. Vinegar Ph Vs Concentration.
From www.researchgate.net
Maintenance of acidic pH after application of pH 3.5 vinegar, pH 3.5 Vinegar Ph Vs Concentration Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Two types of vinegar with equal levels of acidity can also have different ph values. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Vinegar is acidic because of its low ph. Consuming. Vinegar Ph Vs Concentration.
From www.numerade.com
SOLVED Reaction of Vinegar and Baking Soda with Cabbage Juice What Vinegar Ph Vs Concentration Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Ph is the measure of hydrogen ion. On a technical level, vinegar is a solution of acetic. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Vinegar is acidic because of its. Vinegar Ph Vs Concentration.
From www.mometrix.com
pH Overview (Chemistry Review Video) Vinegar Ph Vs Concentration Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. On a technical level, vinegar is a solution of acetic. Two varieties of vinegar with the same level of acidity can have different ph values. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Ph. Vinegar Ph Vs Concentration.
From thoughtfullysustainable.com
How to Explain the Chemistry of Cleaning with Vinegar Thoughtfully Vinegar Ph Vs Concentration Two varieties of vinegar with the same level of acidity can have different ph values. Vinegar is acidic because of its low ph. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. However, adding water to vinegar can never turn vinegar into an. On a technical level, vinegar is a. Vinegar Ph Vs Concentration.
From greengobbler.com
What is the pH of Vinegar? Green Gobbler Vinegar Ph Vs Concentration Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Two types. Vinegar Ph Vs Concentration.
From greatist.com
Understanding the pH of Vinegar Benefits and Uses Explained Vinegar Ph Vs Concentration Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Two types of vinegar with equal levels of acidity can also have different ph values. On a technical level, vinegar is a solution of acetic. However, adding water to vinegar can never turn vinegar into an. Diluting vinegar with water increases its ph. Vinegar Ph Vs Concentration.
From www.slideserve.com
PPT 14. Production of vinegar PowerPoint Presentation, free download Vinegar Ph Vs Concentration Ph is the measure of hydrogen ion. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Two types of vinegar with equal levels of acidity can also have different ph values. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Two varieties. Vinegar Ph Vs Concentration.
From www.artofit.org
Vinegar acidity chart acidity and ph level explained Artofit Vinegar Ph Vs Concentration Two varieties of vinegar with the same level of acidity can have different ph values. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Two types of vinegar with equal levels of acidity can also have different ph values. However, adding water to vinegar can never turn vinegar into an.. Vinegar Ph Vs Concentration.
From www.chegg.com
Solved How to calculate vinegar concentration Vinegar Ph Vs Concentration Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Most vinegars have a ph of 2 to 3 and a strength of 4 to 8 percent. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. However, adding water to vinegar can never. Vinegar Ph Vs Concentration.
From www.researchgate.net
Simple regression and correlation between the concentration of vinegar Vinegar Ph Vs Concentration On a technical level, vinegar is a solution of acetic. Ph is the measure of the hydrogen ion concentration and is easily measured with a simple ph. Two varieties of vinegar with the same level of acidity can have different ph values. However, adding water to vinegar can never turn vinegar into an. Two types of vinegar with equal levels. Vinegar Ph Vs Concentration.
From greengobbler.com
What is the pH of Vinegar? Green Gobbler Vinegar Ph Vs Concentration Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. Vinegar is acidic because of its low ph. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Two types of vinegar with equal levels of acidity can also have different. Vinegar Ph Vs Concentration.
From www.researchgate.net
Efficacy of various undiluted vinegar types (white (pH 2.4), apple Vinegar Ph Vs Concentration Vinegar is acidic because of its low ph. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Two varieties of vinegar with the same level of acidity can have different ph values. Ph is the measure of hydrogen ion. Acetic acid is what distinguishes vinegar and global standards of taste. Vinegar Ph Vs Concentration.
From medium.com
Experiment to Determine the Concentration of Acetic Acid in Vinegar Vinegar Ph Vs Concentration Vinegar is acidic because of its low ph. Diluting vinegar with water increases its ph value, because vinegar is an acid and water has a higher ph level. However, adding water to vinegar can never turn vinegar into an. Ph is the measure of hydrogen ion. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify. Vinegar Ph Vs Concentration.
From www.researchgate.net
Simple regression and correlation between the concentration of vinegar Vinegar Ph Vs Concentration Two varieties of vinegar with the same level of acidity can have different ph values. Acetic acid is what distinguishes vinegar and global standards of taste and safety specify minimum acidity levels for vinegar. Consuming it doesn’t affect your body’s natural ph levels, which stay stable unless you have an underlying medical condition. Ph is the measure of hydrogen ion.. Vinegar Ph Vs Concentration.