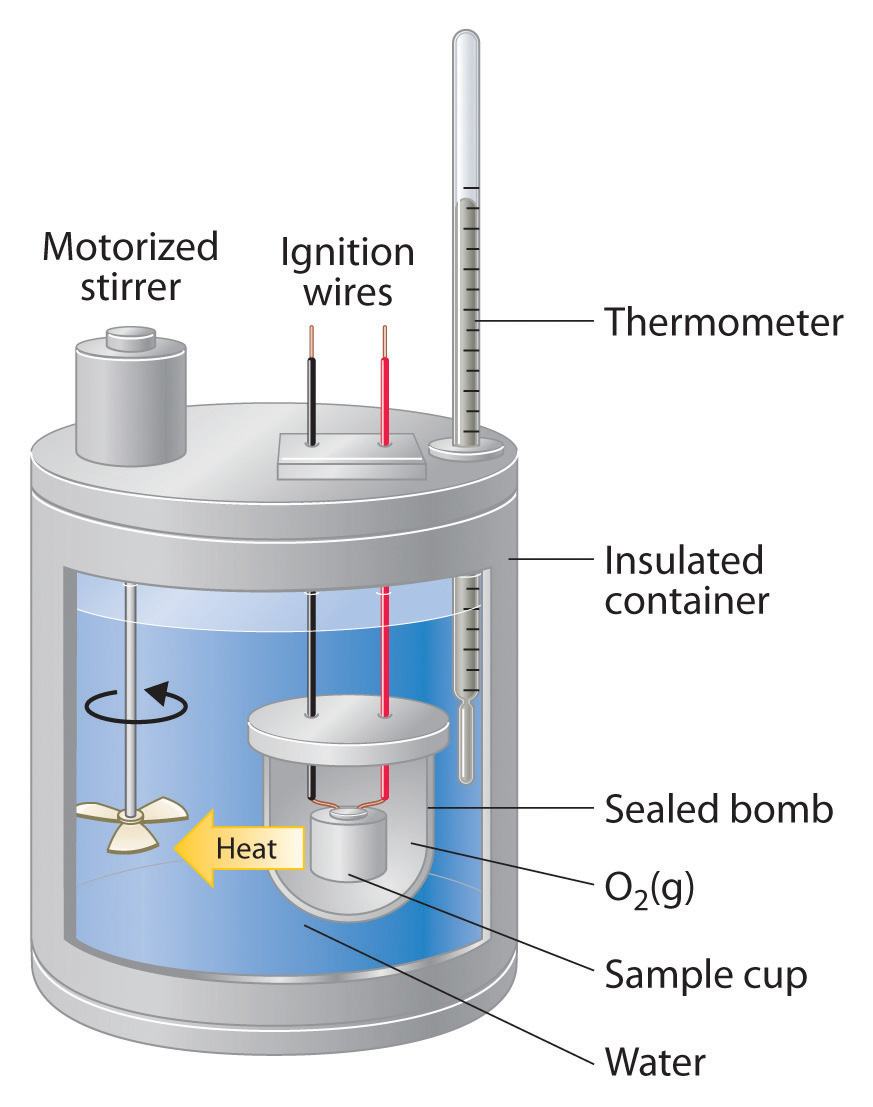
Basic Calorimeter Equation . here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. Since the mass of this water and its temperature change are. in the equations, q is the amount of heat measured in joules. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. It is easily measured, and if. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. M is mass measured in grams. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical.
from saylordotorg.github.io
the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. calorimetry is used to measure amounts of heat transferred to or from a substance. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. To do so, the heat is exchanged with a. Calculate and interpret heat and related properties using typical. M is mass measured in grams. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. It is easily measured, and if.
Calorimetry
Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. It is easily measured, and if. Calculate and interpret heat and related properties using typical. M is mass measured in grams. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. Since the mass of this water and its temperature change are. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Explain the technique of calorimetry. To do so, the heat is exchanged with a. calorimetry is used to measure amounts of heat transferred to or from a substance. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. in the equations, q is the amount of heat measured in joules. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined.
From www.thoughtco.com
Coffee Cup and Bomb Calorimetry Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical. It is easily measured,. Basic Calorimeter Equation.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Explain the technique of calorimetry. Since the mass of this water and its temperature change are. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Calculate and interpret heat. Basic Calorimeter Equation.
From people.chem.umass.edu
to Adobe GoLive 6 Basic Calorimeter Equation Calculate and interpret heat and related properties using typical. To do so, the heat is exchanged with a. Since the mass of this water and its temperature change are. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. calorimetry is used to measure amounts of heat transferred to or from a. Basic Calorimeter Equation.
From www.youtube.com
Principle of Calorimetry YouTube Basic Calorimeter Equation calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate and interpret heat and related properties using typical. To do so, the heat is exchanged with a. It is easily measured, and if. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. the heat. Basic Calorimeter Equation.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Basic Calorimeter Equation M is mass measured in grams. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. Since the mass of this water and its temperature change are. To do so, the heat is exchanged with a. It is easily measured, and if. the heat lost by the pan is. Basic Calorimeter Equation.
From en.ppt-online.org
What is enthalpy? online presentation Basic Calorimeter Equation Explain the technique of calorimetry. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. It is easily measured, and if. M is mass measured in grams. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. describe a simple. Basic Calorimeter Equation.
From physique.ensc-rennes.fr
TP calorimetry Basic Calorimeter Equation To do so, the heat is exchanged with a. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calculate and interpret heat and related properties using typical. calorimetry is used to measure amounts of heat transferred to or from a substance. Since the mass of this. Basic Calorimeter Equation.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Basic Calorimeter Equation To do so, the heat is exchanged with a. It is easily measured, and if. calorimetry is used to measure amounts of heat transferred to or from a substance. in the equations, q is the amount of heat measured in joules. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}.. Basic Calorimeter Equation.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Basic Calorimeter Equation To do so, the heat is exchanged with a. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. calorimetry is used to measure amounts of heat. Basic Calorimeter Equation.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Basic Calorimeter Equation here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. Since the mass of this water and its temperature change are. calorimetry is used to measure amounts of heat transferred to or from a substance. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated. Basic Calorimeter Equation.
From chemistrytalk.org
Calorimetry ChemTalk Basic Calorimeter Equation in the equations, q is the amount of heat measured in joules. Since the mass of this water and its temperature change are. It is easily measured, and if. Explain the technique of calorimetry. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. the heat lost by the pan. Basic Calorimeter Equation.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Basic Calorimeter Equation here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. calorimetry is used to measure amounts of heat transferred to or from a substance. in the equations, q is the amount of heat measured in joules. the heat lost by the pan is equal to the heat. Basic Calorimeter Equation.
From users.highland.edu
Calorimetry Basic Calorimeter Equation It is easily measured, and if. in the equations, q is the amount of heat measured in joules. Explain the technique of calorimetry. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. the heat lost by the pan is equal to the heat gained by the water—that is the. Basic Calorimeter Equation.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Basic Calorimeter Equation calorimetry is used to measure amounts of heat transferred to or from a substance. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. It is easily measured, and. Basic Calorimeter Equation.
From serc.carleton.edu
Calorimetry Basic Calorimeter Equation calorimetry is used to measure amounts of heat transferred to or from a substance. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Since the mass of this water and its temperature change are. here the calorimeter (as in the q calorimeter term) is considered to be the water. Basic Calorimeter Equation.
From socratic.org
Finding DeltaH given heat released and mass in bomb calorimetry? Socratic Basic Calorimeter Equation To do so, the heat is exchanged with a. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. It is easily measured, and if. calorimetry is used to measure amounts of heat transferred to or from a substance. Since the mass of this water and its temperature change are. here. Basic Calorimeter Equation.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Basic Calorimeter Equation To do so, the heat is exchanged with a. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. the heat lost by the pan is equal to the. Basic Calorimeter Equation.
From www.pinterest.com.au
Pin on All About Science Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Since the mass of this water and its temperature change are. the equation above can. Basic Calorimeter Equation.
From ecampusontario.pressbooks.pub
3.5 Calorimétrie La Chimie Générale pour les GeeGees Basic Calorimeter Equation It is easily measured, and if. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. calorimetry is used to measure amounts of heat transferred to or from a substance. in the equations, q is the amount of heat measured in joules. the heat lost by the. Basic Calorimeter Equation.
From study.com
Calorimetry Definition, Equation & Types Lesson Basic Calorimeter Equation Calculate and interpret heat and related properties using typical. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. the heat lost by the pan is equal to the heat gained by. Basic Calorimeter Equation.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Basic Calorimeter Equation calorimetry is used to measure amounts of heat transferred to or from a substance. Explain the technique of calorimetry. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. Since the mass of this water and its temperature change are. Calculate and interpret heat and related properties using typical. To do so,. Basic Calorimeter Equation.
From saylordotorg.github.io
Calorimetry Basic Calorimeter Equation Explain the technique of calorimetry. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Calculate and interpret heat and related properties using typical. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. It is easily measured, and if. the. Basic Calorimeter Equation.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Basic Calorimeter Equation describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. Explain the technique of calorimetry. To do so, the heat is exchanged with a. the heat lost by the pan is. Basic Calorimeter Equation.
From www.youtube.com
CALORIMETRY_Part 01 YouTube Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Explain the technique of calorimetry. To do so, the heat is exchanged with a. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. in. Basic Calorimeter Equation.
From www.chegg.com
Solved Use the Calorimeter Constant and equations provided Basic Calorimeter Equation in the equations, q is the amount of heat measured in joules. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. To do so, the heat is exchanged with a. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of. Basic Calorimeter Equation.
From www.youtube.com
050 Calorimetry YouTube Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. calorimetry is used to measure amounts of heat transferred to or from a substance. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. . Basic Calorimeter Equation.
From mmsphyschem.com
Calorimetry MM's site Basic Calorimeter Equation To do so, the heat is exchanged with a. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. M is mass measured in grams. Since the mass of this water and its temperature change are. in the equations, q is the amount of heat measured in. Basic Calorimeter Equation.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. It is easily measured, and if. the equation above can also be used to calculate \(q_{rxn}\) from \(q_{calorimeter}\) calculated by equation \ref{2a}. Since the mass of this water and its temperature change are. calorimetry is used. Basic Calorimeter Equation.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup.. Basic Calorimeter Equation.
From www.youtube.com
Bomb Calorimeter vs Coffee Cup Calorimeter Problem Constant Pressure Basic Calorimeter Equation the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. in the equations, q is the amount of heat measured in joules. It is easily measured, and if. Explain the technique of calorimetry. here the calorimeter (as in the q calorimeter term) is considered to be. Basic Calorimeter Equation.
From courses.lumenlearning.com
Calorimetry Chemistry Basic Calorimeter Equation Since the mass of this water and its temperature change are. Calculate and interpret heat and related properties using typical. in the equations, q is the amount of heat measured in joules. To do so, the heat is exchanged with a. It is easily measured, and if. the heat lost by the pan is equal to the heat. Basic Calorimeter Equation.
From www.thoughtco.com
Calorimeter Definition in Chemistry Basic Calorimeter Equation To do so, the heat is exchanged with a. in the equations, q is the amount of heat measured in joules. Since the mass of this water and its temperature change are. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. here the calorimeter (as. Basic Calorimeter Equation.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Basic Calorimeter Equation It is easily measured, and if. Calculate and interpret heat and related properties using typical. describe a simple calorimeter and explain how it is employed and how its heat capacity is determined. Explain the technique of calorimetry. Since the mass of this water and its temperature change are. the heat lost by the pan is equal to the. Basic Calorimeter Equation.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.3 Calorimetry翰林国际教育 Basic Calorimeter Equation here the calorimeter (as in the q calorimeter term) is considered to be the water in the coffee cup. Calculate and interpret heat and related properties using typical. To do so, the heat is exchanged with a. Since the mass of this water and its temperature change are. the equation above can also be used to calculate \(q_{rxn}\). Basic Calorimeter Equation.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Basic Calorimeter Equation Since the mass of this water and its temperature change are. To do so, the heat is exchanged with a. Explain the technique of calorimetry. the heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry. Calculate and interpret heat and related properties using typical. here the calorimeter. Basic Calorimeter Equation.