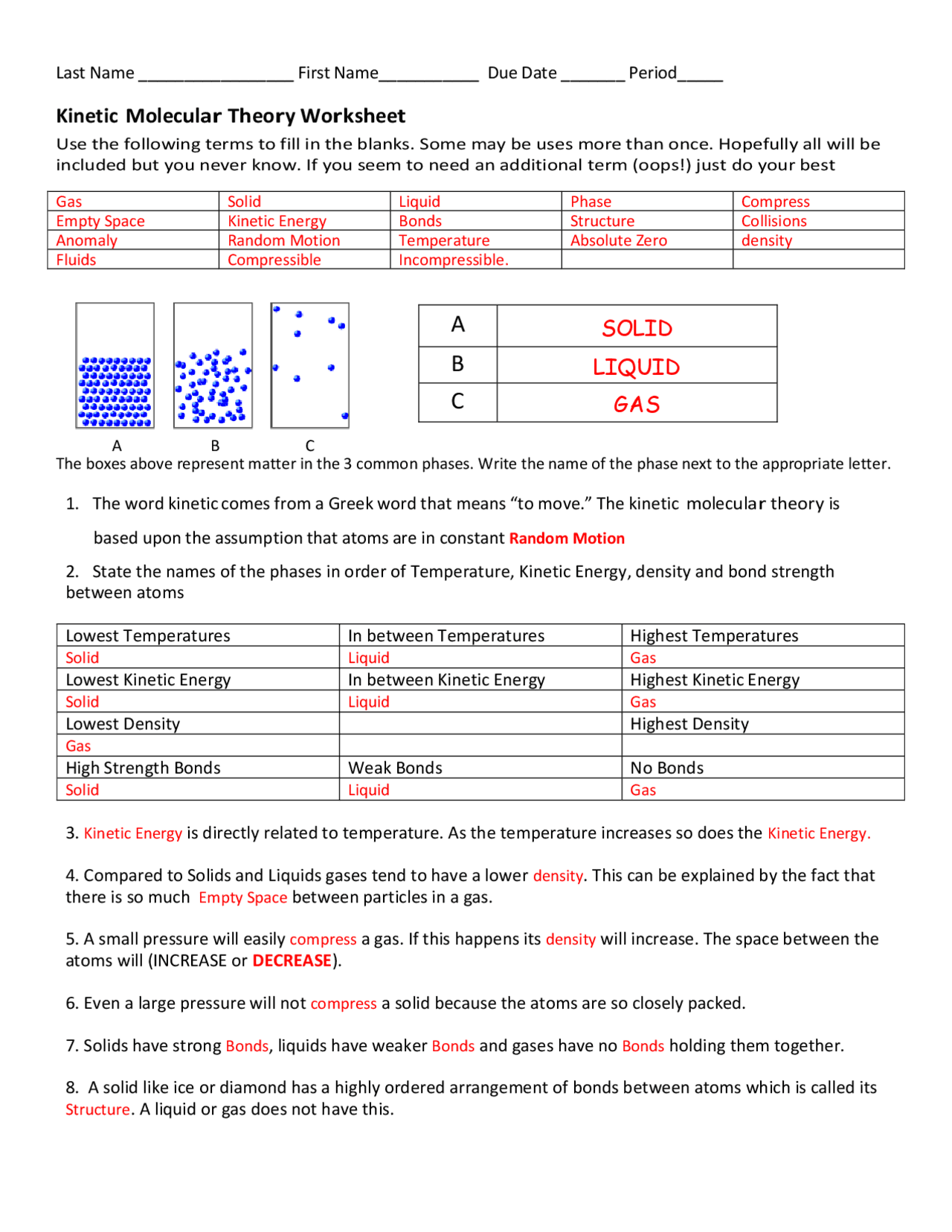
Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices . There are attractive forces between gas molecules,. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. Ke = 1 2 m u 2. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. which of the following statements is true about kinetic molecular theory? Expressing mass in kilograms and. define pressure and describe how gases exert pressure. b) the size of the particle is large compared to the volume. C) the collisions of particles with one another is not completely elastic. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: (b) pressure is due to the collisions of the gas particles with the walls of the container.
from www.docsity.com
5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Expressing mass in kilograms and. There are attractive forces between gas molecules,. define pressure and describe how gases exert pressure. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: b) the size of the particle is large compared to the volume. C) the collisions of particles with one another is not completely elastic. which of the following statements is true about kinetic molecular theory? (b) pressure is due to the collisions of the gas particles with the walls of the container. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr.
Molecular Theory Worksheet Docsity
Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices b) the size of the particle is large compared to the volume. (b) pressure is due to the collisions of the gas particles with the walls of the container. which of the following statements is true about kinetic molecular theory? Expressing mass in kilograms and. b) the size of the particle is large compared to the volume. There are attractive forces between gas molecules,. Ke = 1 2 m u 2. define pressure and describe how gases exert pressure. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: C) the collisions of particles with one another is not completely elastic. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr.
From www.pinterest.com
A 15 question printable molecular theory crossword with answer Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices There are attractive forces between gas molecules,. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. which of the following statements is true about kinetic molecular theory? Ke = 1 2 m u 2. (b) pressure is due to the collisions of the gas particles with the walls of the container. define pressure and. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From novenalunasolitaria.blogspot.com
Molecular Theory Worksheet And Practice Questions worksheet Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices b) the size of the particle is large compared to the volume. define pressure and describe how gases exert pressure. which of the following statements is true about kinetic molecular theory? Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. Ke = 1 2 m u 2. 5.0 (1 review) which statement describes. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From study.com
Quiz & Worksheet Properties of Gases with the Molecular Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices There are attractive forces between gas molecules,. C) the collisions of particles with one another is not completely elastic. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. which of the following statements is true about kinetic molecular theory? Ke = 1 2 m u 2. Convert between units of gas pressure. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studypool.com
SOLUTION 5 the molecular theory Studypool Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. Expressing mass in kilograms and. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: (b) pressure is due to the collisions of the gas particles with the walls of the container. which of the following statements. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.slideserve.com
PPT Theory PowerPoint Presentation, free download Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: define pressure and describe how gases exert pressure. which of the following statements is true about kinetic molecular theory? C) the collisions of particles with one. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studypool.com
SOLUTION Lesson 10 1 molecular theory Studypool Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices which of the following statements is true about kinetic molecular theory? b) the size of the particle is large compared to the volume. define pressure and describe how gases exert pressure. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Ke = 1 2 m u 2. the kinetic. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.englishworksheet.my.id
Molecular Theory Worksheet Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: There are attractive forces between gas molecules,. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. define pressure and describe how gases exert pressure. Expressing mass in kilograms and. C) the collisions of. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studypool.com
SOLUTION Chemistry class notes molecular theory 1 Studypool Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices which of the following statements is true about kinetic molecular theory? Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. (b) pressure is due to the collisions of the gas particles with the walls of the container. b) the size of the particle is large compared to the volume. C) the collisions of particles. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.slideserve.com
PPT The Molecular Theory PowerPoint Presentation, free Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Ke = 1 2 m u 2. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. C) the collisions of particles with one another is not completely elastic. Expressing mass in kilograms and. define pressure and describe how gases. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.pinterest.com
50 Molecular theory Worksheet Chessmuseum Template Library Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices b) the size of the particle is large compared to the volume. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.youtube.com
Theory of Gases YouTube Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Ke = 1 2 m u 2. There are attractive forces between gas molecules,. C) the collisions of particles with one another is not completely elastic. b) the size of the particle is large compared to the volume. Expressing mass in kilograms and. which of the following statements is true about kinetic molecular theory? 5.0 (1 review) which. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.abrigatelapelicula.com
Molecular Theory Concept Map Map of world Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: Expressing mass in kilograms and. define pressure and describe how gases exert pressure. b) the size of the particle is large compared to the volume. There are attractive forces between gas molecules,. which of the following statements is. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studocu.com
The Molecular theory The Molecular theory Explain Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices (b) pressure is due to the collisions of the gas particles with the walls of the container. C) the collisions of particles with one another is not completely elastic. define pressure and describe how gases exert pressure. Ke = 1 2 m u 2. Expressing mass in kilograms and. There are attractive forces between gas molecules,. the kinetic. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.liveworksheets.com
Molecular Theory interactive worksheet Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Expressing mass in kilograms and. C) the collisions of particles with one another is not completely elastic. which of the following statements is true about kinetic molecular theory? (b) pressure is due to the collisions of the gas particles with the walls of the container. There are attractive forces between gas molecules,. b) the size of the particle. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From studylib.net
Chapter 10 Notes The MOLECULAR THEORY is based on Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Ke = 1 2 m u 2. There are attractive forces between gas molecules,. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. C) the collisions of particles with one another is not completely elastic. define pressure and describe how gases exert pressure. 5.0 (1 review) which statement describes the particles of an ideal gas. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.blendspace.com
Molecular Theory Lessons Blendspace Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices (b) pressure is due to the collisions of the gas particles with the walls of the container. Expressing mass in kilograms and. C) the collisions of particles with one another is not completely elastic. define pressure and describe how gases exert pressure. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Ke. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From studylib.net
Molecular Theory Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices which of the following statements is true about kinetic molecular theory? There are attractive forces between gas molecules,. Ke = 1 2 m u 2. Expressing mass in kilograms and. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. define pressure and describe how gases exert pressure. C) the collisions of. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studocu.com
Chemistry Notes Lesson 1 Molecular Theory of Matter Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: define pressure and describe how gases exert pressure. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. There are attractive forces between gas molecules,. b) the size of the particle is large. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studocu.com
molecular theory Molecular Theory The Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices which of the following statements is true about kinetic molecular theory? C) the collisions of particles with one another is not completely elastic. define pressure and describe how gases exert pressure. Expressing mass in kilograms and. Ke = 1 2 m u 2. (b) pressure is due to the collisions of the gas particles with the walls of. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.chegg.com
Solved Which one of the following is NOT a statement of the Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices define pressure and describe how gases exert pressure. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. C) the collisions of particles with one another is not completely elastic. Expressing mass in kilograms and. b) the size of the particle is large compared to the volume. There are attractive forces between. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.coursehero.com
[Solved] Select all that apply. When using the molecular theory Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Ke = 1 2 m u 2. b) the size of the particle is large compared to the volume. Expressing mass in kilograms and. There are attractive forces between gas molecules,. (b) pressure is due to the collisions of the gas particles with the walls of the container. which of the following statements is true about kinetic molecular. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.nagwa.com
Question Video Identifying the True Statement About the VSEPR Theory Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices (b) pressure is due to the collisions of the gas particles with the walls of the container. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. define pressure and describe how gases exert pressure. Expressing mass in kilograms and. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic.. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.semanarioangolense.net
Answer Key Molecular Theory Worksheet Answers » Semanario Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Ke = 1 2 m u 2. define pressure and describe how gases exert pressure. Expressing mass in kilograms and. b) the size of the particle is large compared to the volume. which of the following statements is true about kinetic molecular theory? Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. (b). Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.vrogue.co
Solved Question 17 Point How Does Molecular T vrogue.co Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: C) the collisions of particles with one another is not completely elastic. b) the size of the particle is large compared to the volume.. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.slideserve.com
PPT Gases and the Theory PowerPoint Presentation Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. which of the following statements is true about kinetic molecular theory? define pressure and describe how gases exert pressure. Ke = 1 2 m u 2. b) the size of the particle is large compared to the volume. (b) pressure is due to the. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.chegg.com
Solved Which of the following statements are true regarding Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices (b) pressure is due to the collisions of the gas particles with the walls of the container. define pressure and describe how gases exert pressure. Expressing mass in kilograms and. b) the size of the particle is large compared to the volume. the kinetic energy (ke) of a particle of mass ( m) and speed ( u). Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.chegg.com
Solved Which of the following statements concerning the Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices which of the following statements is true about kinetic molecular theory? (b) pressure is due to the collisions of the gas particles with the walls of the container. C) the collisions of particles with one another is not completely elastic. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. the kinetic. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.sliderbase.com
Molecular Theory Presentation Chemistry Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices (b) pressure is due to the collisions of the gas particles with the walls of the container. There are attractive forces between gas molecules,. b) the size of the particle is large compared to the volume. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. Ke = 1 2 m u 2. Expressing mass in. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studypool.com
SOLUTION Chemistry molecular theory Studypool Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Expressing mass in kilograms and. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. There are attractive forces between gas molecules,. Ke = 1 2 m u 2. b) the size of the. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studypool.com
SOLUTION molecular theory notes Studypool Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Expressing mass in kilograms and. Ke = 1 2 m u 2. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: C) the collisions of particles with one another is not completely elastic. b) the size of the particle is large compared to the volume. Convert between units of. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From studylib.net
Homework Set 101 Molecular Theory Use molecular Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices There are attractive forces between gas molecules,. b) the size of the particle is large compared to the volume. Ke = 1 2 m u 2. which of the following statements is true about kinetic molecular theory? define pressure and describe how gases exert pressure. C) the collisions of particles with one another is not completely elastic.. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From novenalunasolitaria.blogspot.com
Molecular Theory Worksheet And Practice Questions worksheet Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. Ke = 1 2 m u 2. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: Expressing mass in kilograms and. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic.. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.studypool.com
SOLUTION Chemistry molecular theory Studypool Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices C) the collisions of particles with one another is not completely elastic. b) the size of the particle is large compared to the volume. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. Ke = 1 2 m u. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.pinterest.ca
an image of some kind of science activity Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices C) the collisions of particles with one another is not completely elastic. the kinetic energy (ke) of a particle of mass ( m) and speed ( u) is given by: Convert between units of gas pressure ( atm atm, mmhg mm hg, torr. Ke = 1 2 m u 2. (b) pressure is due to the collisions of the. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.
From www.docsity.com
Molecular Theory Worksheet Docsity Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices b) the size of the particle is large compared to the volume. 5.0 (1 review) which statement describes the particles of an ideal gas based on the kinetic. Ke = 1 2 m u 2. Expressing mass in kilograms and. (b) pressure is due to the collisions of the gas particles with the walls of the container. Convert between. Which Statement Is True About Kinetic Molecular Theory Group Of Answer Choices.