Fda Medical Device Listing Guidance . Establishments required to list their. Registration of a device establishment, assignment of a registration. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This list contains the most recent final medical device guidance documents. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Establishment registration & device listing. For a complete listing, please see the guidance. Manufacturers must list their devices with the fda.
from meddev-info.blogspot.com
Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Establishments required to list their. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Establishment registration & device listing. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. For a complete listing, please see the guidance. Manufacturers must list their devices with the fda. This list contains the most recent final medical device guidance documents. Registration of a device establishment, assignment of a registration.
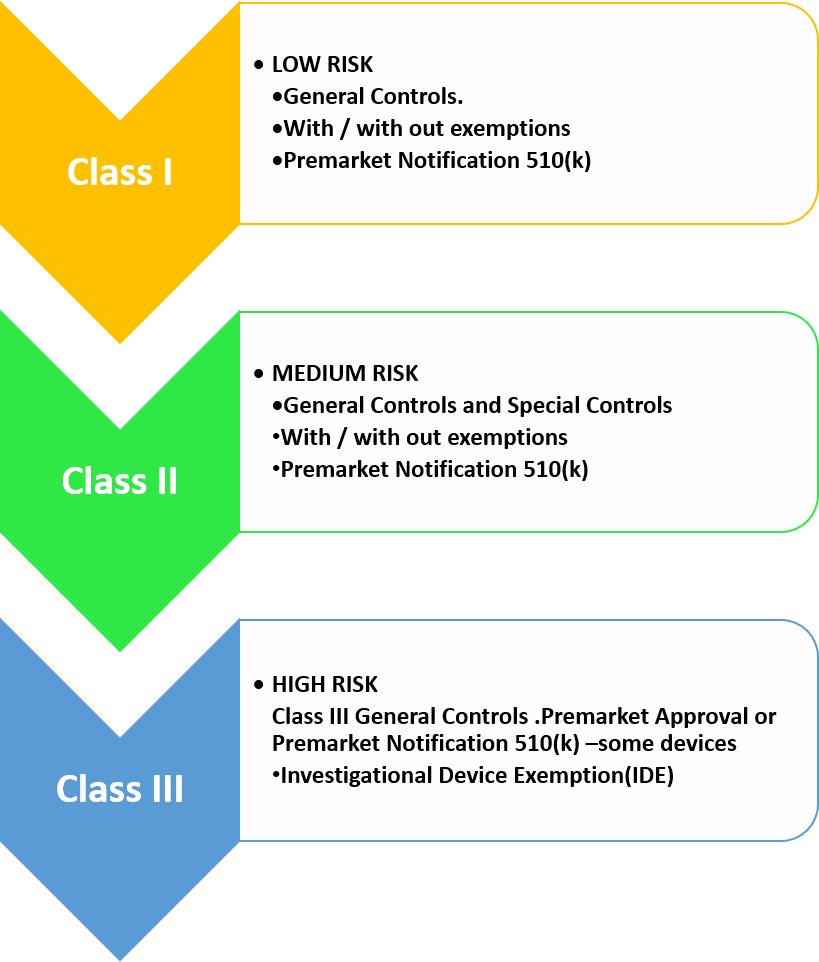
Medical Device Regulation Basics US FDA Medical Device Classification
Fda Medical Device Listing Guidance Registration of a device establishment, assignment of a registration. Registration of a device establishment, assignment of a registration. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Establishments required to list their. Manufacturers must list their devices with the fda. For a complete listing, please see the guidance. This list contains the most recent final medical device guidance documents. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Establishment registration & device listing. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Validation and FDA Review Fda Medical Device Listing Guidance For a complete listing, please see the guidance. This list contains the most recent final medical device guidance documents. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Manufacturers must list their devices with the. Fda Medical Device Listing Guidance.
From medicaldeviceacademy.com
FDA Registration and Listing for Medical Devices Fda Medical Device Listing Guidance Establishments required to list their. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Establishment registration & device listing. Manufacturers must list their devices with the fda. Owners or operators who have been granted a. Fda Medical Device Listing Guidance.
From mavink.com
Fda Medical Device Classification Chart Fda Medical Device Listing Guidance Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration of a device establishment, assignment of a registration. Establishments required to list their. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Labeling regulations pertaining to medical devices are found in the following parts. Fda Medical Device Listing Guidance.
From www.complianceg.com
FDA Medical Device 5 Stage Goals to Launch Medical Devices to Market Fda Medical Device Listing Guidance Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Establishments required to list their. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Establishment registration & device listing. Registration and listing provide the fda with the location of medical. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA on Medical Device Reporting Specific Aspects RegDesk Fda Medical Device Listing Guidance Establishments required to list their. This list contains the most recent final medical device guidance documents. For a complete listing, please see the guidance. Registration of a device establishment, assignment of a registration. Manufacturers must list their devices with the fda. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration and. Fda Medical Device Listing Guidance.
From www.presentationeze.com
FDA Regulatory Requirements Medical Devices.PresentationEZE Fda Medical Device Listing Guidance Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This list contains the most recent final medical device guidance documents. Establishments required to list their. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Establishment registration & device listing.. Fda Medical Device Listing Guidance.
From medicaldeviceacademy.com
Section 513(g)How to Request FDA Device Classification Information Fda Medical Device Listing Guidance For a complete listing, please see the guidance. This list contains the most recent final medical device guidance documents. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Establishments required to list. Fda Medical Device Listing Guidance.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Fda Medical Device Listing Guidance For a complete listing, please see the guidance. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Owners or operators who have been granted a waiver must submit the required. Fda Medical Device Listing Guidance.
From www.access.fda.gov
Register a New Medical Device Facility StepbyStep Instructions Fda Medical Device Listing Guidance Establishment registration & device listing. For a complete listing, please see the guidance. Establishments required to list their. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Owners or operators who have been granted a. Fda Medical Device Listing Guidance.
From www.slideshare.net
FDA Medical Device Guidance Fda Medical Device Listing Guidance Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Establishments required to list their. For a complete listing, please see the guidance. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Guidance documents are prepared for the fda’s staff, regulated industry,. Fda Medical Device Listing Guidance.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Fda Medical Device Listing Guidance Establishments required to list their. For a complete listing, please see the guidance. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Registration of a device establishment, assignment of a registration. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the. Fda Medical Device Listing Guidance.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Medical Device Listing Guidance Establishment registration & device listing. For a complete listing, please see the guidance. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Manufacturers must list their devices with the fda. Labeling regulations pertaining to medical. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA Guidance on Medical Device Reporting Written Procedures, Record Fda Medical Device Listing Guidance Establishments required to list their. Registration of a device establishment, assignment of a registration. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. For a complete listing, please see the guidance. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Fda Medical Device Listing Guidance.
From emmainternational.com
Understanding FDA Guidance Documents Fda Medical Device Listing Guidance Establishments required to list their. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Manufacturers must list their devices with the fda. For a complete listing, please see the guidance. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration and. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA Guidance on Medical Device Reporting Specific Issues Fda Medical Device Listing Guidance This list contains the most recent final medical device guidance documents. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Establishments required to list their. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration of a device establishment,. Fda Medical Device Listing Guidance.
From meddevice.blogspot.com
Medical Device Blog 510(k) Infographic Fda Medical Device Listing Guidance Establishments required to list their. This list contains the most recent final medical device guidance documents. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration of a device establishment, assignment of a registration. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code. Fda Medical Device Listing Guidance.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Fda Medical Device Listing Guidance Manufacturers must list their devices with the fda. Establishments required to list their. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Establishment registration & device listing. Registration and listing provide the. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA Guidance on Least Burdensome Provisions Overview RegDesk Fda Medical Device Listing Guidance Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. For a complete listing, please see the guidance. This list contains the most recent final medical device guidance documents. Establishments required to list their. Establishment registration. Fda Medical Device Listing Guidance.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Fda Medical Device Listing Guidance Establishments required to list their. Manufacturers must list their devices with the fda. Establishment registration & device listing. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. This list contains. Fda Medical Device Listing Guidance.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Fda Medical Device Listing Guidance For a complete listing, please see the guidance. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Labeling regulations pertaining to medical devices are found in the following parts of title 21. Fda Medical Device Listing Guidance.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Fda Medical Device Listing Guidance Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. For a complete listing, please see the guidance. Registration of a device establishment, assignment of a registration. This list contains the most recent final medical device. Fda Medical Device Listing Guidance.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Fda Medical Device Listing Guidance Registration of a device establishment, assignment of a registration. For a complete listing, please see the guidance. Establishments required to list their. Establishment registration & device listing. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. This list contains the most recent final medical device guidance documents. Registration and listing provide the. Fda Medical Device Listing Guidance.
From mavink.com
Fda Medical Device Classification Chart Fda Medical Device Listing Guidance For a complete listing, please see the guidance. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Establishment registration & device listing. This list contains the most recent final medical device guidance documents. Establishments required to list their. Registration and listing provide the fda with the location of medical. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA Guidance on Medical Device Data Systems and Image Devices RegDesk Fda Medical Device Listing Guidance Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Manufacturers must list their devices with the fda. For a complete listing, please see the guidance. Registration of a device establishment, assignment of a registration. Owners or operators who have been granted a waiver must submit the required device listing information, including information. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Medical Device Listing Guidance For a complete listing, please see the guidance. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Establishment registration & device listing. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Registration and listing provide the. Fda Medical Device Listing Guidance.
From diaztradelaw.com
Tips on FDA's Medical Device Registration Process Customs Fda Medical Device Listing Guidance Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Registration of a device establishment, assignment of a registration. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. This list contains the most recent final medical device guidance documents. For. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA Guidance on eCopy Program for Medical Device Submissions RegDesk Fda Medical Device Listing Guidance Establishment registration & device listing. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Registration of a device establishment, assignment of a registration. Guidance documents are prepared for the fda’s. Fda Medical Device Listing Guidance.
From operonstrategist.com
US FDA Medical Device Establishment Registration and Device Listing Fda Medical Device Listing Guidance Establishments required to list their. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). For a complete listing, please see the guidance. Manufacturers must list their devices. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Medical Device Listing Guidance Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. Registration of a device establishment, assignment of a registration. This list contains the most recent final medical device guidance documents. For a complete listing, please see the guidance. Manufacturers must list their devices with the fda. Labeling regulations pertaining to medical devices are. Fda Medical Device Listing Guidance.
From aaos.org
An Overview of the FDA Approval Process for Devices Fda Medical Device Listing Guidance Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. Establishments required to list their. Establishment registration & device listing. This list contains the most recent final medical device guidance documents. Registration of a device establishment, assignment of a registration. Registration and listing provide the fda with the location of. Fda Medical Device Listing Guidance.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Medical Device Listing Guidance Registration of a device establishment, assignment of a registration. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Establishments required to list their. This list contains the most recent final medical device guidance documents. Manufacturers must list their devices with the fda. Establishment registration & device listing.. Fda Medical Device Listing Guidance.
From www.access.fda.gov
Device Registration and Listing Module (DRLM) StepbyStep Instructions Fda Medical Device Listing Guidance Registration of a device establishment, assignment of a registration. This list contains the most recent final medical device guidance documents. Manufacturers must list their devices with the fda. Establishment registration & device listing. Guidance documents are prepared for the fda’s staff, regulated industry, and the public to describe the fda’s. Labeling regulations pertaining to medical devices are found in the. Fda Medical Device Listing Guidance.
From www.greenlight.guru
Understanding the FDA Medical Device Classification System Fda Medical Device Listing Guidance Registration of a device establishment, assignment of a registration. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. This list contains the most recent final medical device guidance documents. Establishment registration & device listing. Registration and listing provide the fda with the location of medical device establishments and the. Fda Medical Device Listing Guidance.
From www.scribd.com
FDA Guidance 510 k Checklist Federal Food Medical Device Fda Medical Device Listing Guidance Establishments required to list their. This list contains the most recent final medical device guidance documents. Registration and listing provide the fda with the location of medical device establishments and the devices manufactured. For a complete listing, please see the guidance. Establishment registration & device listing. Registration of a device establishment, assignment of a registration. Guidance documents are prepared for. Fda Medical Device Listing Guidance.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Fda Medical Device Listing Guidance Manufacturers must list their devices with the fda. Owners or operators who have been granted a waiver must submit the required device listing information, including information required by. This list contains the most recent final medical device guidance documents. Establishment registration & device listing. For a complete listing, please see the guidance. Guidance documents are prepared for the fda’s staff,. Fda Medical Device Listing Guidance.