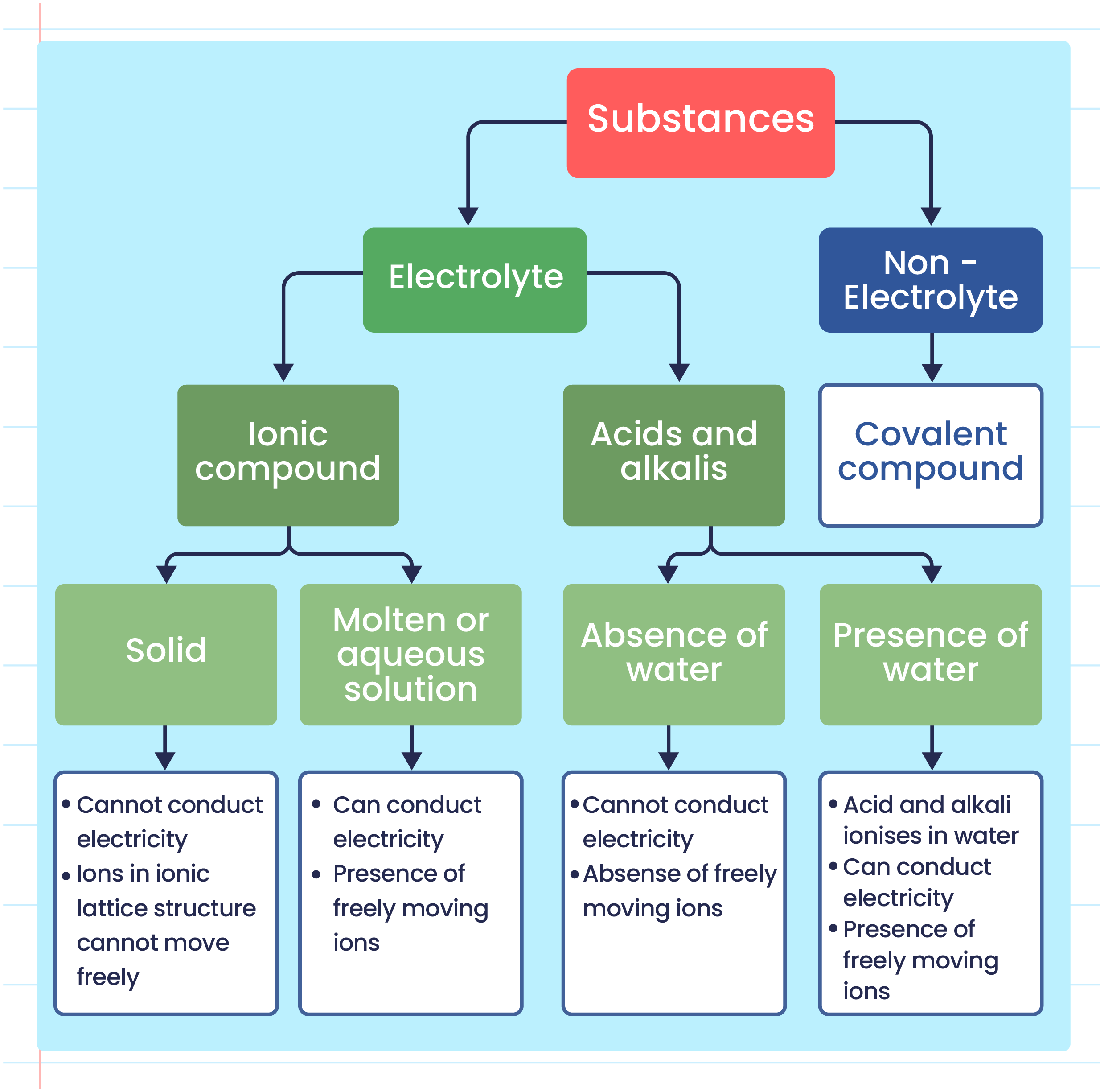
Electrolyte Solution Examples . Distinguish between the physical and chemical changes that accompany dissolution. Define electrolyte and give examples of electrolytes. Weak electrolytes partially ionize in water. Pretty much any dissociation into. For example, \ (\ce {nh4oh}\). Define and give examples of electrolytes. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Define and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. By the end of this section, you will be able to: Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Strong acids, strong bases, and salts are strong electrolytes. Some neutral molecules are present in their solutions.
from pandai.me
Strong acids, strong bases, and salts are strong electrolytes. Pretty much any dissociation into. By the end of this section, you will be able to: Define and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Define electrolyte and give examples of electrolytes. Some neutral molecules are present in their solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. For example, \ (\ce {nh4oh}\).
Electrolytic Cell
Electrolyte Solution Examples By the end of this section, you will be able to: Define electrolyte and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution. By the end of this section, you will be able to: Define and give examples of electrolytes. For example, \ (\ce {nh4oh}\). Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Define and give examples of electrolytes. Strong acids, strong bases, and salts are strong electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Pretty much any dissociation into. Weak electrolytes partially ionize in water. Some neutral molecules are present in their solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water.
From www.narodnatribuna.info
Nonelectrolyte Examples Electrolyte Solution Examples Define and give examples of electrolytes. Some neutral molecules are present in their solutions. Define and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. By the end of this section, you will be able to: Distinguish between the physical and chemical. Electrolyte Solution Examples.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Electrolyte Solution Examples Distinguish between the physical and chemical changes that accompany dissolution. Some neutral molecules are present in their solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Define and give examples of electrolytes. Pretty much any dissociation into. By the end of this section, you will be able to: Distinguish between the physical and chemical changes that accompany. Electrolyte Solution Examples.
From gamesmartz.com
Weak Electrolyte Definition & Image GameSmartz Electrolyte Solution Examples Define and give examples of electrolytes. Strong acids, strong bases, and salts are strong electrolytes. Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Some neutral molecules are present in their solutions. By the end of this section,. Electrolyte Solution Examples.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Electrolyte Solution Examples Weak electrolytes partially ionize in water. Distinguish between the physical and chemical changes that accompany dissolution. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Some neutral molecules are present in their solutions. Define and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution,. Electrolyte Solution Examples.
From www.shutterstock.com
4 Nonelectrolyte 이미지, 스톡 사진 및 벡터 Shutterstock Electrolyte Solution Examples For example, \ (\ce {nh4oh}\). Strong acids, strong bases, and salts are strong electrolytes. Some neutral molecules are present in their solutions. By the end of this section, you will be able to: Pretty much any dissociation into. Define and give examples of electrolytes. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Distinguish between the physical and. Electrolyte Solution Examples.
From www.pinterest.co.uk
What is Electrolysis and Electrolyte with examples. Chemistry lessons Electrolyte Solution Examples Some neutral molecules are present in their solutions. Distinguish between the physical and chemical changes that accompany dissolution. Strong acids, strong bases, and salts are strong electrolytes. For example, \ (\ce {nh4oh}\). Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Small fractions of weak electrolytes'. Electrolyte Solution Examples.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Electrolyte Solution Examples Define and give examples of electrolytes. Small fractions of weak electrolytes' molecules ionize when dissolve in water. By the end of this section, you will be able to: Some neutral molecules are present in their solutions. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Distinguish. Electrolyte Solution Examples.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips Electrolyte Solution Examples Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. By the end of this section, you will be able to: Define and give examples of electrolytes. Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution. Strong acids, strong bases,. Electrolyte Solution Examples.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Electrolyte Solution Examples Strong acids, strong bases, and salts are strong electrolytes. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Some neutral molecules are present in their solutions. Distinguish between the physical and chemical changes that accompany dissolution. Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Weak. Electrolyte Solution Examples.
From www.meritnation.com
Explain electrolytes Science Chemical Effects of Electric Current Electrolyte Solution Examples Small fractions of weak electrolytes' molecules ionize when dissolve in water. Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution. Pretty much any dissociation into. For example, \ (\ce {nh4oh}\). Define and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the. Electrolyte Solution Examples.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps Electrolyte Solution Examples Weak electrolytes partially ionize in water. Pretty much any dissociation into. By the end of this section, you will be able to: Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. For example, \ (\ce {nh4oh}\). Distinguish between the physical and chemical changes that accompany dissolution. Define and. Electrolyte Solution Examples.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Electrolyte Solution Examples By the end of this section, you will be able to: For example, \ (\ce {nh4oh}\). Define and give examples of electrolytes. Weak electrolytes partially ionize in water. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Define electrolyte and give examples of electrolytes. Pretty much any dissociation into. Define and give examples of. Electrolyte Solution Examples.
From www.w3schools.blog
Strong and weak electrolytes W3schools Electrolyte Solution Examples Define and give examples of electrolytes. Strong acids, strong bases, and salts are strong electrolytes. Define and give examples of electrolytes. Weak electrolytes partially ionize in water. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Pretty much any dissociation into. Some neutral molecules are present in their solutions. By the end of this section, you will be. Electrolyte Solution Examples.
From www.pinterest.es
To Identify Given Solutions as Electrolyte and Non Electrolyte NEB Electrolyte Solution Examples By the end of this section, you will be able to: Define and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Distinguish between the physical and chemical changes that accompany dissolution. Strong acids, strong bases, and salts are strong electrolytes. Some. Electrolyte Solution Examples.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy Electrolyte Solution Examples Strong acids, strong bases, and salts are strong electrolytes. Some neutral molecules are present in their solutions. Weak electrolytes partially ionize in water. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. For example, \ (\ce {nh4oh}\). Small fractions of weak electrolytes' molecules ionize when dissolve. Electrolyte Solution Examples.
From www.studypool.com
SOLUTION Differences of Electrolyte and NonElectrolyte solutions and Electrolyte Solution Examples By the end of this section, you will be able to: Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Strong acids, strong bases, and salts are strong electrolytes. Define and give examples of electrolytes. Define and give examples of electrolytes. For example, \ (\ce {nh4oh}\). Some neutral molecules are present in their solutions.. Electrolyte Solution Examples.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrolyte Solution Examples Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution. Strong acids, strong bases, and salts are strong electrolytes. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Weak electrolytes partially ionize in water. Pretty much any dissociation into. Some neutral molecules are present in their solutions. Define and give examples of. Electrolyte Solution Examples.
From www.ck12.org
Solvation, Dissociation, and Electrolytes Example 4 ( Video Electrolyte Solution Examples Small fractions of weak electrolytes' molecules ionize when dissolve in water. Weak electrolytes partially ionize in water. By the end of this section, you will be able to: Define and give examples of electrolytes. Define electrolyte and give examples of electrolytes. Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution. Placing a salt. Electrolyte Solution Examples.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Electrolyte Solution Examples Small fractions of weak electrolytes' molecules ionize when dissolve in water. Strong acids, strong bases, and salts are strong electrolytes. Pretty much any dissociation into. Some neutral molecules are present in their solutions. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Distinguish between the physical. Electrolyte Solution Examples.
From www.slideshare.net
6.1 meaning of electrolyte Electrolyte Solution Examples For example, \ (\ce {nh4oh}\). Small fractions of weak electrolytes' molecules ionize when dissolve in water. Distinguish between the physical and chemical changes that accompany dissolution. By the end of this section, you will be able to: Define electrolyte and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Pretty. Electrolyte Solution Examples.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Electrolyte Solution Examples Some neutral molecules are present in their solutions. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Define and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Strong acids, strong bases, and salts are strong. Electrolyte Solution Examples.
From www.slideserve.com
PPT Chemistry 101 Chap. 4 PowerPoint Presentation, free download Electrolyte Solution Examples Define and give examples of electrolytes. Some neutral molecules are present in their solutions. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Distinguish between the physical and chemical changes that accompany dissolution. Define electrolyte and give examples of electrolytes. Small fractions of weak electrolytes' molecules. Electrolyte Solution Examples.
From ecurrencythailand.com
Which Is The Strongest Electrolyte? 10 Most Correct Answers Electrolyte Solution Examples For example, \ (\ce {nh4oh}\). Define and give examples of electrolytes. Weak electrolytes partially ionize in water. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Distinguish between the physical and chemical changes that accompany dissolution. Strong acids, strong bases, and salts are strong electrolytes. Define. Electrolyte Solution Examples.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL Electrolyte Solution Examples Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Strong acids, strong bases, and salts are strong electrolytes. For example, \ (\ce {nh4oh}\). Some neutral molecules are present in their solutions. By the end of this section, you will be able to: Define electrolyte and give examples of electrolytes. Small fractions of weak electrolytes'. Electrolyte Solution Examples.
From www.slideserve.com
PPT SOLUTIONS OF ELECTROLYTES PowerPoint Presentation, free download Electrolyte Solution Examples Some neutral molecules are present in their solutions. Weak electrolytes partially ionize in water. By the end of this section, you will be able to: Define and give examples of electrolytes. Pretty much any dissociation into. Strong acids, strong bases, and salts are strong electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes.. Electrolyte Solution Examples.
From hayden-relbrewer.blogspot.com
HaydenRelBrewer Electrolyte Solution Examples Pretty much any dissociation into. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Distinguish between the physical and chemical changes that accompany dissolution. Weak electrolytes partially ionize in water. By the end of this section, you will be able to: Define and. Electrolyte Solution Examples.
From pandai.me
Electrolytic Cell Electrolyte Solution Examples Small fractions of weak electrolytes' molecules ionize when dissolve in water. For example, \ (\ce {nh4oh}\). Define electrolyte and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. Pretty much any dissociation into. Some neutral molecules are present in their solutions. Distinguish. Electrolyte Solution Examples.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Electrolyte Solution Examples For example, \ (\ce {nh4oh}\). Pretty much any dissociation into. Weak electrolytes partially ionize in water. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. By the end of this section, you will be able to: Strong acids, strong bases, and salts are strong electrolytes. Define. Electrolyte Solution Examples.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrolyte Solution Examples Weak electrolytes partially ionize in water. Some neutral molecules are present in their solutions. Distinguish between the physical and chemical changes that accompany dissolution. Pretty much any dissociation into. For example, \ (\ce {nh4oh}\). Define and give examples of electrolytes. Strong acids, strong bases, and salts are strong electrolytes. Placing a salt in a liquid (such as water) often contributes. Electrolyte Solution Examples.
From www.pathwaystochemistry.com
Electrolytes and Nonelectrolytes Pathways to Chemistry Electrolyte Solution Examples By the end of this section, you will be able to: Weak electrolytes partially ionize in water. For example, \ (\ce {nh4oh}\). Some neutral molecules are present in their solutions. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Pretty much any dissociation into. Define electrolyte and give examples of electrolytes. Distinguish between the physical and chemical changes. Electrolyte Solution Examples.
From www.youtube.com
Examples of Electrolyte and Nonelectrolyte Solution YouTube Electrolyte Solution Examples For example, \ (\ce {nh4oh}\). Weak electrolytes partially ionize in water. Strong acids, strong bases, and salts are strong electrolytes. Define electrolyte and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt. Electrolyte Solution Examples.
From quizizz.com
Electrolysis of Aqueous Solutions 222 plays Quizizz Electrolyte Solution Examples Define and give examples of electrolytes. Define and give examples of electrolytes. Define electrolyte and give examples of electrolytes. Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes. Weak electrolytes partially ionize in water. Distinguish between the physical and chemical changes that accompany dissolution. For example, \ (\ce {nh4oh}\). Small fractions of weak electrolytes'. Electrolyte Solution Examples.
From www.slideserve.com
PPT Solutions Review Pt 2 PowerPoint Presentation, free download ID Electrolyte Solution Examples Define electrolyte and give examples of electrolytes. Placing a salt in a liquid (such as water) often contributes to an electrolyte solution, as the salt components dissociate in a process. By the end of this section, you will be able to: Strong acids, strong bases, and salts are strong electrolytes. Distinguish between the physical and chemical changes that accompany dissolution.. Electrolyte Solution Examples.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrolyte Solution Examples Distinguish between the physical and chemical changes that accompany dissolution. By the end of this section, you will be able to: Define electrolyte and give examples of electrolytes. Define and give examples of electrolytes. Small fractions of weak electrolytes' molecules ionize when dissolve in water. Some neutral molecules are present in their solutions. Weak electrolytes partially ionize in water. For. Electrolyte Solution Examples.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry Electrolyte Solution Examples Pretty much any dissociation into. Some neutral molecules are present in their solutions. For example, \ (\ce {nh4oh}\). Strong acids, strong bases, and salts are strong electrolytes. Define electrolyte and give examples of electrolytes. By the end of this section, you will be able to: Distinguish between the physical and chemical changes that accompany dissolution of ionic and covalent electrolytes.. Electrolyte Solution Examples.