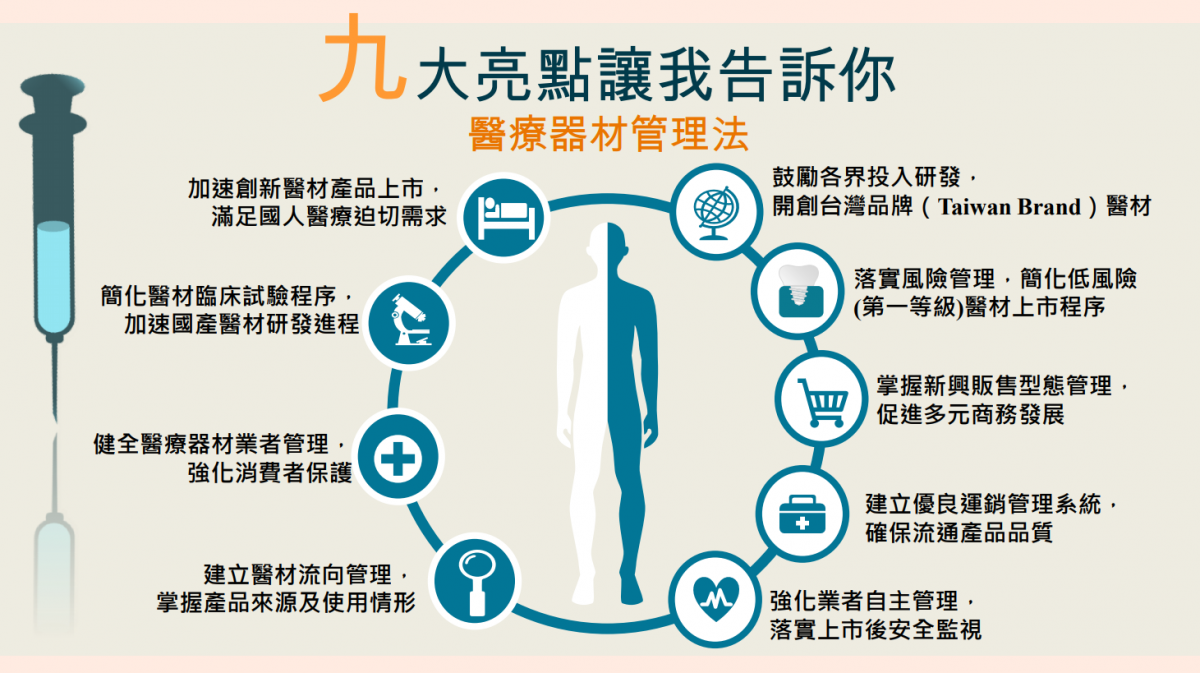
Medical Device Management Act Taiwan . To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This independent law helps to build a. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians • reg. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. • medical device quality management system regulations • reg.
from biomed.taiwan.gov.tw
• medical device quality management system regulations • reg. For management of medical devices technicians • reg. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. The medical devices act governing medical devices officially took effect on may 1, 2021. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). This independent law helps to build a.
BioMed Taiwan
Medical Device Management Act Taiwan The medical devices act governing medical devices officially took effect on may 1, 2021. • medical device quality management system regulations • reg. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians • reg. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. This independent law helps to build a. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the.
From mdcpublishers.com
Health Medical Device Act 2012 Medical Device Management Act Taiwan • medical device quality management system regulations • reg. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This independent law helps to build a. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. The medical devices act governing medical devices officially. Medical Device Management Act Taiwan.
From healthcare-international.meti.go.jp
MEDICAL DEVICE ACT 2012 (ACT 737)(UPDATES ON MEDICAL DEVICE ACT Medical Device Management Act Taiwan This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. • medical device quality management system regulations • reg. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. The medical devices act (mda) takes effect from may 1st, 2021. Medical Device Management Act Taiwan.
From www.joharidigital.com
Importance of Quality Management System in Medical Device Manufacturing Medical Device Management Act Taiwan • medical device quality management system regulations • reg. This independent law helps to build a. The medical devices act governing medical devices officially took effect on may 1, 2021. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes. Medical Device Management Act Taiwan.
From igmpi.ac.in
Medical Device Management Medical Device Management Act Taiwan The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians •. Medical Device Management Act Taiwan.
From www.medicaltaiwan.com.tw
MEDICAL TAIWAN Medical Device Management Act Taiwan The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). • medical device quality management system regulations • reg. For management of medical devices technicians • reg. This independent law helps to build a. The medical devices act will establish a system to effectively regulate medical devices throughout the. Medical Device Management Act Taiwan.
From shotnelo.weebly.com
Medical device risk assessment template shotnelo Medical Device Management Act Taiwan This independent law helps to build a. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. For management of medical devices technicians • reg. To supervise. Medical Device Management Act Taiwan.
From healthcare-international.meti.go.jp
MEDICAL DEVICE ACT 2012 (ACT 737)(UPDATES ON MEDICAL DEVICE ACT Medical Device Management Act Taiwan The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote. Medical Device Management Act Taiwan.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Management Act Taiwan The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians • reg. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. This independent. Medical Device Management Act Taiwan.
From www.idconic.com
Importing of General Medical Devices idcOnic Co., Ltd. Medical Device Management Act Taiwan This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). • medical device quality management system regulations • reg. The medical devices act governing medical devices officially. Medical Device Management Act Taiwan.
From www.med-license.com
Taiwan TFDA Medical Device Registration Service License Biomaterial Medical Device Management Act Taiwan • medical device quality management system regulations • reg. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. The medical devices act (mda) takes effect from may 1st, 2021. Medical Device Management Act Taiwan.
From www.linkedin.com
Adapting to Taiwan's New Medical Device Act Navigating Changes Medical Device Management Act Taiwan The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). • medical device quality management system regulations • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians • reg. The medical devices act will establish a system. Medical Device Management Act Taiwan.
From www.pacificbridgemedical.com
Taiwan Medical Device Registration TFDA Approval Medical Device Management Act Taiwan This independent law helps to build a. For management of medical devices technicians • reg. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. The medical devices act will. Medical Device Management Act Taiwan.
From www.arqon.com
Thai FDA Medical Device Act (2nd edition) Medical Device Management Act Taiwan This independent law helps to build a. • medical device quality management system regulations • reg. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. The medical devices act governing. Medical Device Management Act Taiwan.
From www.scribd.com
Common Submission Dossier Malaysia Medical Device Act 2012 (ACT 737 Medical Device Management Act Taiwan For management of medical devices technicians • reg. This independent law helps to build a. The medical devices act governing medical devices officially took effect on may 1, 2021. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. • medical device quality management system regulations • reg. The medical devices act. Medical Device Management Act Taiwan.
From credevo.com
Regulatory scenario & approval process for clinical trials in Taiwan Medical Device Management Act Taiwan The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. • medical device quality management system regulations • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. This independent law helps to build a. For management of medical devices technicians • reg. To supervise the management. Medical Device Management Act Taiwan.
From www.orielstat.com
What is a Medical Device Quality Management System (QMS)? Medical Device Management Act Taiwan The medical devices act governing medical devices officially took effect on may 1, 2021. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This independent law helps to build. Medical Device Management Act Taiwan.
From www.artixio.com
FAQs Taiwan (TFDA) Regulations for Medical Device Registration Medical Device Management Act Taiwan The medical devices act governing medical devices officially took effect on may 1, 2021. • medical device quality management system regulations • reg. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). To supervise the management of medical devices, by considering the potential risk of certain medical devices,. Medical Device Management Act Taiwan.
From es.slideshare.net
Taiwan medical device registration and approval chart EMERGO Medical Device Management Act Taiwan The medical devices act governing medical devices officially took effect on may 1, 2021. • medical device quality management system regulations • reg. This independent law helps to build a. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This act is established to ensure the safety, effectiveness, and quality of. Medical Device Management Act Taiwan.
From www.youtube.com
Medical Device Regulatory in Asia_Taiwan YouTube Medical Device Management Act Taiwan For management of medical devices technicians • reg. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. The medical devices act governing medical devices officially took effect on may 1, 2021. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal. Medical Device Management Act Taiwan.
From healthcare-international.meti.go.jp
MEDICAL DEVICE ACT 2012 (ACT 737)(UPDATES ON MEDICAL DEVICE ACT Medical Device Management Act Taiwan This independent law helps to build a. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). • medical device quality management system regulations • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians • reg. This. Medical Device Management Act Taiwan.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Device Management Act Taiwan This independent law helps to build a. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. • medical device quality management system regulations • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. This act is established to ensure the safety, effectiveness, and quality of. Medical Device Management Act Taiwan.
From www.slideshare.net
Taiwan medical device registration and approval chart EMERGO Medical Device Management Act Taiwan • medical device quality management system regulations • reg. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians • reg. The medical devices act (mda) takes effect from may 1st, 2021. Medical Device Management Act Taiwan.
From www.medicaldesignandoutsourcing.com
Medical Taiwan 2023 Digital health, innovation, and sustainability Medical Device Management Act Taiwan To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. The medical. Medical Device Management Act Taiwan.
From enterprisepeak.com
Medical Devices from Concept to Commercialization Enterprise Peak Medical Device Management Act Taiwan The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. • medical device quality management system regulations • reg. This independent law helps to build a. The medical devices act. Medical Device Management Act Taiwan.
From www.visgeneer.com
Certificates Medical Device Taiwan Leader in IVDs, Skincare Medical Device Management Act Taiwan • medical device quality management system regulations • reg. For management of medical devices technicians • reg. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. This independent law. Medical Device Management Act Taiwan.
From www.slideteam.net
Medical Device Development And Commercialization Process PPT PowerPoint Medical Device Management Act Taiwan The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. This independent law helps to build a. • medical device quality management system regulations • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. To supervise the management of medical devices, by considering the potential risk. Medical Device Management Act Taiwan.
From paper.li
EBME Newsletter Medical Device Management Act Taiwan This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. This independent law helps to build a. To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. For management of medical devices technicians • reg. The medical devices act (mda). Medical Device Management Act Taiwan.
From biomed.taiwan.gov.tw
BioMed Taiwan Medical Device Management Act Taiwan This independent law helps to build a. For management of medical devices technicians • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. The medical devices act will establish a system to. Medical Device Management Act Taiwan.
From www.regdesk.co
MHRA Guidance on Medical Software and Applications RegDesk Medical Device Management Act Taiwan The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. • medical device quality management system regulations • reg. The medical devices act governing medical devices officially. Medical Device Management Act Taiwan.
From issuu.com
Investigating The Leading Medical Device Distributors in Taiwan by Medical Device Management Act Taiwan The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). • medical device quality management system regulations • reg. For management of medical devices technicians • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. The medical devices act will establish a system. Medical Device Management Act Taiwan.
From pharmadocx.com
Quality Control in the Medical Device Assembly Process Pharmadocx Medical Device Management Act Taiwan The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22 regulations and 16 legal orders). The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. • medical device quality management system regulations • reg. For management of medical devices technicians • reg. This act is established. Medical Device Management Act Taiwan.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Medical Device Management Act Taiwan To supervise the management of medical devices, by considering the potential risk of certain medical devices, the competent. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. • medical device quality management system regulations • reg. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of. Medical Device Management Act Taiwan.
From www.scribd.com
Introduction To Medical Device Act 2012 (Act 737) and Medical Device Medical Device Management Act Taiwan This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. The medical devices act governing medical devices officially took effect on may 1, 2021. For management of medical devices technicians • reg. The medical devices act (mda) takes effect from may 1st, 2021 (authorizes the announcement of 22. Medical Device Management Act Taiwan.
From chinameddevice.com
Medical Device Clinical Trial Phases in China A StepbyStep Guide Medical Device Management Act Taiwan This act is established to ensure the safety, effectiveness, and quality of medical devices to be used by citizens, to promote the. • medical device quality management system regulations • reg. The medical devices act governing medical devices officially took effect on may 1, 2021. The medical devices act will establish a system to effectively regulate medical devices throughout the. Medical Device Management Act Taiwan.
From www.scribd.com
Medical Device Act & Authority Act 2012 (ACT 737 & 738) PDF Medical Device Management Act Taiwan For management of medical devices technicians • reg. The medical devices act will establish a system to effectively regulate medical devices throughout the medical device life. • medical device quality management system regulations • reg. This independent law helps to build a. The medical devices act governing medical devices officially took effect on may 1, 2021. The medical devices act. Medical Device Management Act Taiwan.