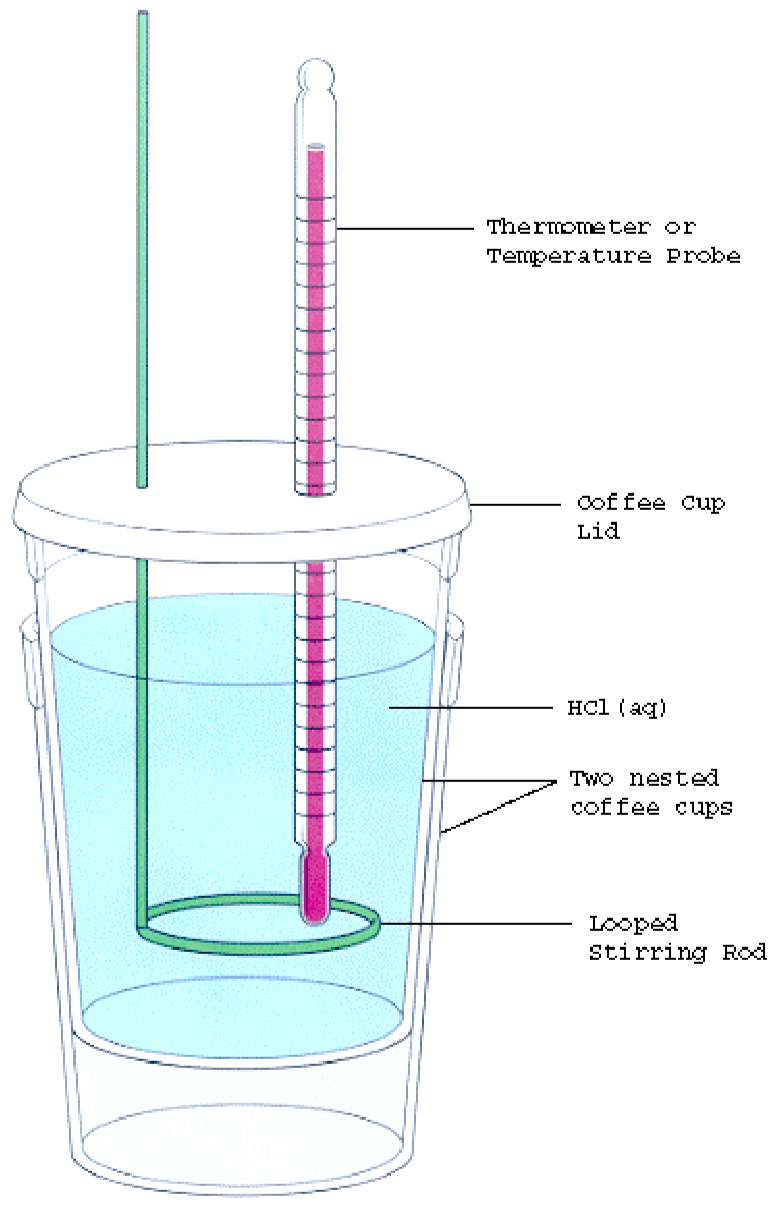
Thermochemistry Calorimetry Lab . What is the specific heat of the metal? Read this text, which describes the two. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. Calorimetry is used to measure amounts of heat transferred to. It shows you how to calculate the quantity of heat transferred using specific heat capacity. The process of measuring heat based on observing the temperature change when a. There are two main types of calorimetry: As part of this lab, you will: When the contents of the calorimeter are. The device used to measure heat flow is called a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known.
from chem.libretexts.org
Read this text, which describes the two. The process of measuring heat based on observing the temperature change when a. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. There are two main types of calorimetry: What is the specific heat of the metal? The device used to measure heat flow is called a calorimeter. It shows you how to calculate the quantity of heat transferred using specific heat capacity. As part of this lab, you will: Calorimetry is used to measure amounts of heat transferred to.
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Thermochemistry Calorimetry Lab Read this text, which describes the two. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. It shows you how to calculate the quantity of heat transferred using specific heat capacity. Calorimetry is used to measure amounts of heat transferred to. As part of this lab, you will: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. The process of measuring heat based on observing the temperature change when a. What is the specific heat of the metal? The device used to measure heat flow is called a calorimeter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Read this text, which describes the two. There are two main types of calorimetry: When the contents of the calorimeter are.
From www.pinterest.com.au
Pin on Science Friday Thermochemistry Calorimetry Lab Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. There are two main types of calorimetry: As part of this lab, you will: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. It shows you how to calculate the. Thermochemistry Calorimetry Lab.
From answerschoolkane101.z21.web.core.windows.net
Calorimetry Pogil Worksheet Answer Key Thermochemistry Calorimetry Lab There are two main types of calorimetry: When the contents of the calorimeter are. Calorimetry is used to measure amounts of heat transferred to. The device used to measure heat flow is called a calorimeter. As part of this lab, you will: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. The process of measuring heat based. Thermochemistry Calorimetry Lab.
From studylib.net
experiment 9 Thermochemistry Calorimetry Thermochemistry Calorimetry Lab The device used to measure heat flow is called a calorimeter. Calorimetry is used to measure amounts of heat transferred to. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. What is the specific heat of the metal? Constant pressure, or coffee. Thermochemistry Calorimetry Lab.
From courses.lumenlearning.com
Calorimetry CHEM 1305 Introductory Chemistry Thermochemistry Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. There are two main types of calorimetry: Calorimetry is used to measure amounts of heat transferred to. As part of this lab, you will: It shows you how to calculate the quantity of heat transferred using specific heat. Thermochemistry Calorimetry Lab.
From notexchange.com.au
Chemistry Thermochemistry & Calorimetry Notes NoteXchange Thermochemistry Calorimetry Lab Read this text, which describes the two. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. The device used to measure heat flow is called a calorimeter. As part of this lab, you will: Calorimetry. Thermochemistry Calorimetry Lab.
From www.youtube.com
Thermochemistry Calorimetry Lab YouTube Thermochemistry Calorimetry Lab Calorimetry is used to measure amounts of heat transferred to. The process of measuring heat based on observing the temperature change when a. What is the specific heat of the metal? Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. As part of this lab, you will: In this experiment you will heat a known mass of. Thermochemistry Calorimetry Lab.
From www.chegg.com
Solved EXPERIMENT 7 Thermochemistry Calorimetry and Hess's Thermochemistry Calorimetry Lab Read this text, which describes the two. What is the specific heat of the metal? It shows you how to calculate the quantity of heat transferred using specific heat capacity. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. There are two main types of calorimetry: One technique we can use to measure the amount of heat. Thermochemistry Calorimetry Lab.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Thermochemistry Calorimetry Lab When the contents of the calorimeter are. As part of this lab, you will: Read this text, which describes the two. It shows you how to calculate the quantity of heat transferred using specific heat capacity. What is the specific heat of the metal? Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. There are two main. Thermochemistry Calorimetry Lab.
From wisc.pb.unizin.org
Day 27 Thermochemistry and Enthalpy Chemistry 109, Fall 2020 Thermochemistry Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The process of measuring heat based on observing the temperature change when a. The device used to measure heat flow is called a calorimeter. What is the specific heat of the metal? Read this text, which describes the. Thermochemistry Calorimetry Lab.
From www.chegg.com
Solved Thermochemistry Calorimetry Pre Lab Given the Thermochemistry Calorimetry Lab What is the specific heat of the metal? In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is used to measure amounts of heat transferred to. It shows you how to calculate the quantity of heat transferred using specific heat capacity.. Thermochemistry Calorimetry Lab.
From www.scribd.com
Calorimetry Lab Explanation Chemistry Physical Sciences Free 30 Thermochemistry Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. As part of this lab, you will: There are two main types of calorimetry: When the contents of the calorimeter are. It shows you how to calculate the quantity of heat transferred using specific heat capacity. What is. Thermochemistry Calorimetry Lab.
From www.studocu.com
Lab 17 Calorimetry and Thermochemistry “stealing joules” CHEM 106 Thermochemistry Calorimetry Lab In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is used to measure amounts of heat transferred to. The process of measuring heat based on observing the temperature change when a. There are two main types of calorimetry: What is the. Thermochemistry Calorimetry Lab.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Thermochemistry Calorimetry Lab The process of measuring heat based on observing the temperature change when a. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. There are two main types of calorimetry: When the contents of the calorimeter are. Calorimetry is used to measure amounts. Thermochemistry Calorimetry Lab.
From www.chegg.com
Solved Thermochemistry Calorimetry and Hess's Law PRELAB Thermochemistry Calorimetry Lab Read this text, which describes the two. It shows you how to calculate the quantity of heat transferred using specific heat capacity. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. There are two main types of calorimetry: When the contents of. Thermochemistry Calorimetry Lab.
From www.studocu.com
Lab 2 CHM1311 Grade A+ Worksheet Experiment 2 Thermochemistry Thermochemistry Calorimetry Lab What is the specific heat of the metal? There are two main types of calorimetry: The process of measuring heat based on observing the temperature change when a. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. As part of this lab,. Thermochemistry Calorimetry Lab.
From www.youtube.com
Thermochemistry Enthalpy and Coffee Cup Calorimeter. YouTube Thermochemistry Calorimetry Lab In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. The device used to measure heat flow is called a calorimeter. The process of measuring heat based on observing the temperature change when a. As part of this lab, you will: It shows. Thermochemistry Calorimetry Lab.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Thermochemistry Calorimetry Lab Read this text, which describes the two. Calorimetry is used to measure amounts of heat transferred to. As part of this lab, you will: There are two main types of calorimetry: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. The process of measuring heat based on observing the temperature change when a. In this experiment you. Thermochemistry Calorimetry Lab.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Thermochemistry Calorimetry Lab It shows you how to calculate the quantity of heat transferred using specific heat capacity. As part of this lab, you will: Calorimetry is used to measure amounts of heat transferred to. There are two main types of calorimetry: When the contents of the calorimeter are. The device used to measure heat flow is called a calorimeter. In this experiment. Thermochemistry Calorimetry Lab.
From giogtyufh.blob.core.windows.net
Calorimetry And Thermochemistry at Steven Stephens blog Thermochemistry Calorimetry Lab It shows you how to calculate the quantity of heat transferred using specific heat capacity. Calorimetry is used to measure amounts of heat transferred to. Read this text, which describes the two. What is the specific heat of the metal? There are two main types of calorimetry: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. One. Thermochemistry Calorimetry Lab.
From www.studocu.com
Thermochemistry and Calorimetry Measuring Heat Changes in Chemical Thermochemistry Calorimetry Lab In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. The device used to measure heat flow is called a calorimeter. What is the specific heat of the metal? Calorimetry is used to measure amounts of heat transferred to. Constant pressure, or coffee. Thermochemistry Calorimetry Lab.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Thermochemistry Calorimetry Lab There are two main types of calorimetry: As part of this lab, you will: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. It shows you how to calculate the quantity of heat transferred using specific heat capacity. What is the specific heat of the metal? The device used to measure heat flow is called a calorimeter.. Thermochemistry Calorimetry Lab.
From www.chegg.com
Thermochemistry lab Thermochemistry Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Read this text, which describes the two. There are two main types of calorimetry: The device used to measure heat flow is called a calorimeter. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. The. Thermochemistry Calorimetry Lab.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Thermochemistry Calorimetry Lab Calorimetry is used to measure amounts of heat transferred to. The process of measuring heat based on observing the temperature change when a. When the contents of the calorimeter are. As part of this lab, you will: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It. Thermochemistry Calorimetry Lab.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Thermochemistry Calorimetry Lab As part of this lab, you will: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. It shows you how to calculate the quantity of heat transferred using specific heat. Thermochemistry Calorimetry Lab.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Thermochemistry Calorimetry Lab It shows you how to calculate the quantity of heat transferred using specific heat capacity. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. When the contents of the calorimeter are. What is the specific. Thermochemistry Calorimetry Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Thermochemistry Calorimetry Lab It shows you how to calculate the quantity of heat transferred using specific heat capacity. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. Calorimetry is used to measure amounts of heat transferred to. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter. Thermochemistry Calorimetry Lab.
From socratic.org
Question 5d3f9 + Example Thermochemistry Calorimetry Lab Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. What is the specific heat of the metal? Calorimetry is used to measure amounts of heat transferred to. The device used to measure heat flow is called a calorimeter. When the contents of the calorimeter are. It shows you how to calculate the quantity of heat transferred using. Thermochemistry Calorimetry Lab.
From www.chegg.com
Solved 4ICHM151LL THERMOCHEMISTRY Lab Report III Magnesium Thermochemistry Calorimetry Lab One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The device used to measure heat flow is called a calorimeter. There are two main types of calorimetry: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. It shows you how to calculate the quantity. Thermochemistry Calorimetry Lab.
From atanchem.weebly.com
AP Chem Chemistry LHS Thermochemistry Calorimetry Lab When the contents of the calorimeter are. As part of this lab, you will: Calorimetry is used to measure amounts of heat transferred to. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. It shows you how to calculate the quantity of heat transferred using specific heat capacity. In this experiment you will heat a known mass. Thermochemistry Calorimetry Lab.
From www.studocu.com
Thermochemistry and Calorimetry Lab Answer CHM 111 NOVA Studocu Thermochemistry Calorimetry Lab It shows you how to calculate the quantity of heat transferred using specific heat capacity. When the contents of the calorimeter are. The process of measuring heat based on observing the temperature change when a. There are two main types of calorimetry: The device used to measure heat flow is called a calorimeter. Read this text, which describes the two.. Thermochemistry Calorimetry Lab.
From www.youtube.com
Thermochemistry Calorimetry Introduction YouTube Thermochemistry Calorimetry Lab It shows you how to calculate the quantity of heat transferred using specific heat capacity. Read this text, which describes the two. The process of measuring heat based on observing the temperature change when a. What is the specific heat of the metal? In this experiment you will heat a known mass of a metal to a known temperature and. Thermochemistry Calorimetry Lab.
From studylib.net
Lab 33 calorimetry lab Thermochemistry Calorimetry Lab It shows you how to calculate the quantity of heat transferred using specific heat capacity. Calorimetry is used to measure amounts of heat transferred to. As part of this lab, you will: Read this text, which describes the two. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Thermochemistry Calorimetry Lab.
From www.studocu.com
Lab Report 17 + 18 Lab 17 Calorimetry and Thermochemistry “Stealing Thermochemistry Calorimetry Lab Calorimetry is used to measure amounts of heat transferred to. The device used to measure heat flow is called a calorimeter. As part of this lab, you will: It shows you how to calculate the quantity of heat transferred using specific heat capacity. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. One technique we can use. Thermochemistry Calorimetry Lab.
From www.coursehero.com
[Solved] Thermochemistry Calorimetry Pre Lab Given the following Thermochemistry Calorimetry Lab When the contents of the calorimeter are. As part of this lab, you will: The device used to measure heat flow is called a calorimeter. What is the specific heat of the metal? Read this text, which describes the two. It shows you how to calculate the quantity of heat transferred using specific heat capacity. One technique we can use. Thermochemistry Calorimetry Lab.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Thermochemistry Calorimetry Lab The device used to measure heat flow is called a calorimeter. The process of measuring heat based on observing the temperature change when a. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. When the. Thermochemistry Calorimetry Lab.