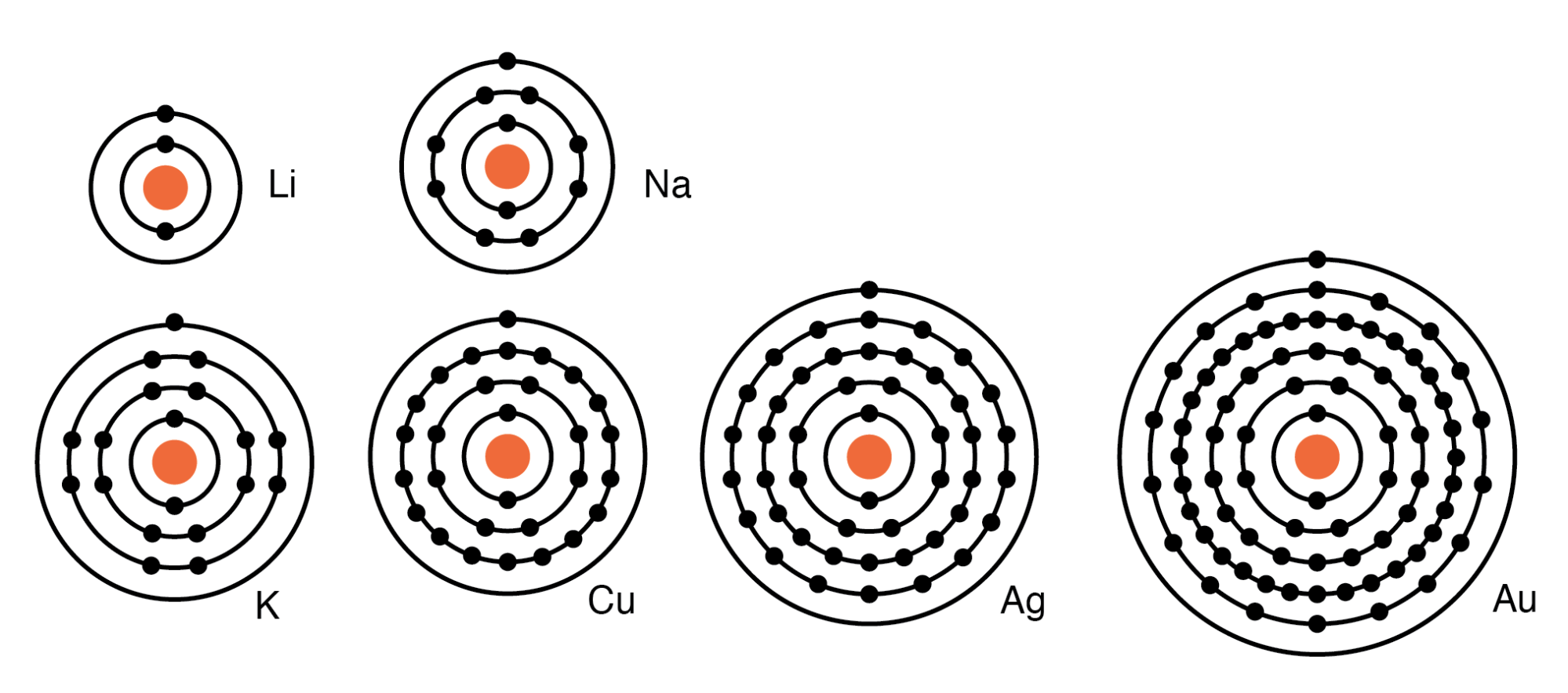
What Is The Outer Shell Electron Configuration In Group 1 . As you will see, this is reflected in important. The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. Electrons in atoms occupy energy levels, also called electron shells, outside the. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Different shells can hold different. The outer electron gets further from the nucleus; To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Note that down each group, the configuration is often similar. When we come to the alkali. When this happens, 1+ ions are formed. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell.
from www.technocrazed.com
The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). As you will see, this is reflected in important. The outer electron gets further from the nucleus; When this happens, 1+ ions are formed. Different shells can hold different. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Note that down each group, the configuration is often similar. When we come to the alkali. Electrons in atoms occupy energy levels, also called electron shells, outside the.
2.3 Valence and Crystal Structure
What Is The Outer Shell Electron Configuration In Group 1 The outer electron gets further from the nucleus; When this happens, 1+ ions are formed. Electrons in atoms occupy energy levels, also called electron shells, outside the. Note that down each group, the configuration is often similar. Different shells can hold different. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. As you will see, this is reflected in important. The outer electron gets further from the nucleus; To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. When we come to the alkali. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13).
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Is The Outer Shell Electron Configuration In Group 1 Different shells can hold different. The outer electron gets further from the nucleus; When this happens, 1+ ions are formed. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Note that down each group, the configuration is often similar. To write the electron configuration of an. What Is The Outer Shell Electron Configuration In Group 1.
From courses.lumenlearning.com
Electrons Biology for Majors I What Is The Outer Shell Electron Configuration In Group 1 Note that down each group, the configuration is often similar. Electrons in atoms occupy energy levels, also called electron shells, outside the. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. To write the electron configuration of an atom, identify the energy level of interest and. What Is The Outer Shell Electron Configuration In Group 1.
From sciencenotes.org
List of Electron Configurations of Elements What Is The Outer Shell Electron Configuration In Group 1 To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: As you will see, this is reflected in important. Electrons in atoms occupy energy levels, also called electron shells, outside the. Within each column, each element has the same valence electron. What Is The Outer Shell Electron Configuration In Group 1.
From www.technocrazed.com
2.3 Valence and Crystal Structure What Is The Outer Shell Electron Configuration In Group 1 When we come to the alkali. Different shells can hold different. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). The outer electron gets further from the nucleus; The atoms of all group 1 elements have similar chemical properties and reactions because they all have one. What Is The Outer Shell Electron Configuration In Group 1.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry What Is The Outer Shell Electron Configuration In Group 1 Electrons in atoms occupy energy levels, also called electron shells, outside the. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. When we come to the alkali. When this happens, 1+ ions are formed. Note that down each group, the configuration is often similar. Different shells. What Is The Outer Shell Electron Configuration In Group 1.
From wou.edu
Ch105 Chapter 2 Atoms, Elements and The Periodic Table Chemistry What Is The Outer Shell Electron Configuration In Group 1 Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. As you will see, this is reflected in important. To write the electron configuration. What Is The Outer Shell Electron Configuration In Group 1.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost What Is The Outer Shell Electron Configuration In Group 1 When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. Electrons in atoms occupy energy levels, also called electron shells, outside the. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as. What Is The Outer Shell Electron Configuration In Group 1.
From chem.libretexts.org
6.4 Electronic Structure of Atoms (Electron Configurations What Is The Outer Shell Electron Configuration In Group 1 When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. The outer electron gets further from the nucleus; When this happens, 1+ ions are formed. Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different. Within each column, each element. What Is The Outer Shell Electron Configuration In Group 1.
From boisestate.pressbooks.pub
3.4 Electronic Structure of Atoms (Electron Configurations) General What Is The Outer Shell Electron Configuration In Group 1 When this happens, 1+ ions are formed. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Note that down each group, the configuration is often similar. When a group 1 element reacts its atoms only need to lose electron, as. What Is The Outer Shell Electron Configuration In Group 1.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is The Outer Shell Electron Configuration In Group 1 Different shells can hold different. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The outer electron gets further from the nucleus; When a group 1 element reacts its atoms only need to lose electron, as there is only 1. What Is The Outer Shell Electron Configuration In Group 1.
From mavink.com
Valence Electron Orbital Diagram What Is The Outer Shell Electron Configuration In Group 1 Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different. Note that down each group, the configuration is often similar. To write the electron configuration of an atom, identify the. What Is The Outer Shell Electron Configuration In Group 1.
From www.youtube.com
Electron shells Elements 118 YouTube What Is The Outer Shell Electron Configuration In Group 1 Electrons in atoms occupy energy levels, also called electron shells, outside the. The outer electron gets further from the nucleus; When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. To write the electron configuration of an atom, identify the energy level of interest and write the. What Is The Outer Shell Electron Configuration In Group 1.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Is The Outer Shell Electron Configuration In Group 1 The outer electron gets further from the nucleus; When this happens, 1+ ions are formed. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different. When a group 1 element. What Is The Outer Shell Electron Configuration In Group 1.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Is The Outer Shell Electron Configuration In Group 1 Note that down each group, the configuration is often similar. When we come to the alkali. When this happens, 1+ ions are formed. Different shells can hold different. Electrons in atoms occupy energy levels, also called electron shells, outside the. The outer electron gets further from the nucleus; Within each column, each element has the same valence electron configuration—for example,. What Is The Outer Shell Electron Configuration In Group 1.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram What Is The Outer Shell Electron Configuration In Group 1 The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. To write the electron configuration of an atom, identify the energy level of interest. What Is The Outer Shell Electron Configuration In Group 1.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png What Is The Outer Shell Electron Configuration In Group 1 When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. Electrons in atoms occupy energy levels, also called electron shells, outside the. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as. What Is The Outer Shell Electron Configuration In Group 1.
From www.newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards What Is The Outer Shell Electron Configuration In Group 1 Note that down each group, the configuration is often similar. Electrons in atoms occupy energy levels, also called electron shells, outside the. As you will see, this is reflected in important. When this happens, 1+ ions are formed. When we come to the alkali. The outer electron gets further from the nucleus; Different shells can hold different. The atoms of. What Is The Outer Shell Electron Configuration In Group 1.
From commons.wikipedia.org
FileElectron shell 082 lead.png Wikimedia Commons What Is The Outer Shell Electron Configuration In Group 1 When we come to the alkali. Electrons in atoms occupy energy levels, also called electron shells, outside the. As you will see, this is reflected in important. When this happens, 1+ ions are formed. The outer electron gets further from the nucleus; To write the electron configuration of an atom, identify the energy level of interest and write the number. What Is The Outer Shell Electron Configuration In Group 1.
From carmanchewchem.blogspot.com
Chemistry Atomic Structure Valency What Is The Outer Shell Electron Configuration In Group 1 To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Note that down each group, the configuration is often similar. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1. What Is The Outer Shell Electron Configuration In Group 1.
From www.thoughtco.com
Electron Configuration Chart What Is The Outer Shell Electron Configuration In Group 1 Electrons in atoms occupy energy levels, also called electron shells, outside the. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). When this. What Is The Outer Shell Electron Configuration In Group 1.
From www.nagwa.com
Question Video Determining What Forms When a Sodium Atom Loses One What Is The Outer Shell Electron Configuration In Group 1 When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: When this happens, 1+ ions are formed. Note. What Is The Outer Shell Electron Configuration In Group 1.
From keystagewiki.com
Electron Orbital Key Stage Wiki What Is The Outer Shell Electron Configuration In Group 1 Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). When this happens, 1+ ions are formed. As you will see, this is reflected in important. Electrons in atoms occupy energy levels, also called electron shells, outside the. The outer electron gets further from the nucleus; The. What Is The Outer Shell Electron Configuration In Group 1.
From www.youtube.com
Electron Shells and the Periodic Table IGCSE Chemistry YouTube What Is The Outer Shell Electron Configuration In Group 1 Different shells can hold different. The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. Note that down each group, the configuration is often similar. When we come to the alkali. When this happens, 1+ ions are formed. When a group 1 element reacts its atoms only. What Is The Outer Shell Electron Configuration In Group 1.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is The Outer Shell Electron Configuration In Group 1 The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. Note that down each group, the configuration is often similar. The outer electron gets further from the nucleus; As you will see, this is reflected in important. Electrons in atoms occupy energy levels, also called electron shells,. What Is The Outer Shell Electron Configuration In Group 1.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind What Is The Outer Shell Electron Configuration In Group 1 Note that down each group, the configuration is often similar. Different shells can hold different. When this happens, 1+ ions are formed. The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. When we come to the alkali. The outer electron gets further from the nucleus; As. What Is The Outer Shell Electron Configuration In Group 1.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is The Outer Shell Electron Configuration In Group 1 To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). When we come to the alkali. When this. What Is The Outer Shell Electron Configuration In Group 1.
From sciencenotes.org
Periodic Table Showing Shells What Is The Outer Shell Electron Configuration In Group 1 When we come to the alkali. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. To write the electron configuration of an atom,. What Is The Outer Shell Electron Configuration In Group 1.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Is The Outer Shell Electron Configuration In Group 1 As you will see, this is reflected in important. Note that down each group, the configuration is often similar. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: Within each column, each element has the same valence electron configuration—for example,. What Is The Outer Shell Electron Configuration In Group 1.
From www.britannica.com
Electron shell Definition & Facts Britannica What Is The Outer Shell Electron Configuration In Group 1 As you will see, this is reflected in important. Note that down each group, the configuration is often similar. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). When a group 1 element reacts its atoms only need to lose electron, as there is only 1. What Is The Outer Shell Electron Configuration In Group 1.
From www.britannica.com
Periodic table Elements, Groups, Properties Britannica What Is The Outer Shell Electron Configuration In Group 1 To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: The outer electron gets further from the nucleus; Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13).. What Is The Outer Shell Electron Configuration In Group 1.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Is The Outer Shell Electron Configuration In Group 1 The outer electron gets further from the nucleus; Note that down each group, the configuration is often similar. Different shells can hold different. To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: When we come to the alkali. Electrons in. What Is The Outer Shell Electron Configuration In Group 1.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy What Is The Outer Shell Electron Configuration In Group 1 To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. When this happens, 1+ ions are formed. As. What Is The Outer Shell Electron Configuration In Group 1.
From www.animalia-life.club
Electron Configuration Of Krypton What Is The Outer Shell Electron Configuration In Group 1 Note that down each group, the configuration is often similar. When this happens, 1+ ions are formed. The outer electron gets further from the nucleus; Electrons in atoms occupy energy levels, also called electron shells, outside the. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell.. What Is The Outer Shell Electron Configuration In Group 1.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Is The Outer Shell Electron Configuration In Group 1 As you will see, this is reflected in important. Different shells can hold different. Electrons in atoms occupy energy levels, also called electron shells, outside the. Within each column, each element has the same valence electron configuration—for example, ns 1 (group 1) or ns 2 np 1 (group 13). When we come to the alkali. The atoms of all group. What Is The Outer Shell Electron Configuration In Group 1.
From www.scienceabc.com
Octet Rule Definition, Explanation, Exceptions And Examples What Is The Outer Shell Electron Configuration In Group 1 Note that down each group, the configuration is often similar. As you will see, this is reflected in important. Electrons in atoms occupy energy levels, also called electron shells, outside the. When a group 1 element reacts its atoms only need to lose electron, as there is only 1 electron in the outer shell. Different shells can hold different. When. What Is The Outer Shell Electron Configuration In Group 1.