Do Elements Keep Or Lose Their Original Properties . the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. All of these elements display several other. the elements in the periodic table are arranged in order of increasing atomic number. The separation of components can be easily done. discuss the relationships between matter, mass, elements, compounds, atoms, and. the components of a mixture each keep their original properties. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. In a mixture, the components do not lose their individual properties. whereas a compound may have very different properties from the elements that compose it, in mixtures the. retention of individual properties:
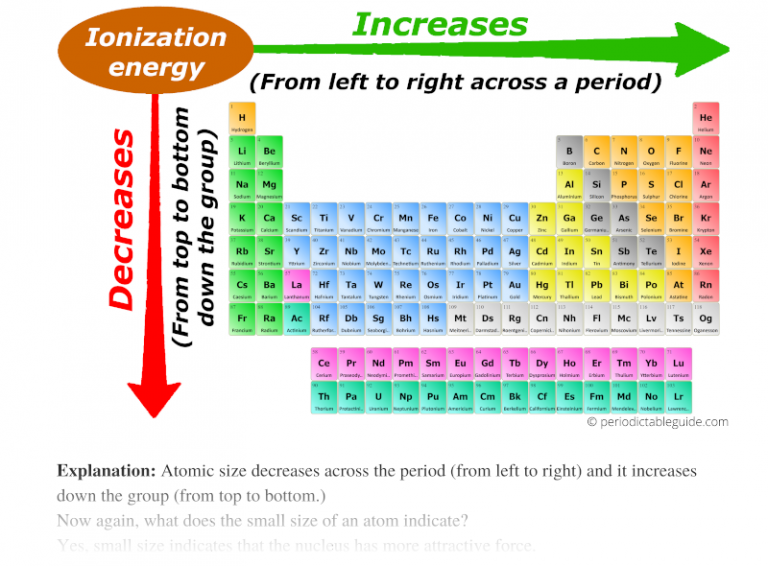
from periodictableguide.com
The separation of components can be easily done. the elements in the periodic table are arranged in order of increasing atomic number. whereas a compound may have very different properties from the elements that compose it, in mixtures the. All of these elements display several other. retention of individual properties: the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. the components of a mixture each keep their original properties. discuss the relationships between matter, mass, elements, compounds, atoms, and. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. In a mixture, the components do not lose their individual properties.
All Periodic Trends in Periodic Table (Explained with Image)
Do Elements Keep Or Lose Their Original Properties the elements in the periodic table are arranged in order of increasing atomic number. The separation of components can be easily done. discuss the relationships between matter, mass, elements, compounds, atoms, and. All of these elements display several other. In a mixture, the components do not lose their individual properties. the elements in the periodic table are arranged in order of increasing atomic number. retention of individual properties: the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. the components of a mixture each keep their original properties. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. whereas a compound may have very different properties from the elements that compose it, in mixtures the.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Do Elements Keep Or Lose Their Original Properties compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. In a mixture, the components do not lose their individual properties. discuss the relationships between matter, mass, elements, compounds, atoms, and. retention of individual properties: whereas a compound may have very different properties from the elements that. Do Elements Keep Or Lose Their Original Properties.
From thechemistryclub.blogspot.com
The Chemistry Club Group 2 Elements Do Elements Keep Or Lose Their Original Properties The separation of components can be easily done. whereas a compound may have very different properties from the elements that compose it, in mixtures the. retention of individual properties: the elements in the periodic table are arranged in order of increasing atomic number. In a mixture, the components do not lose their individual properties. discuss the. Do Elements Keep Or Lose Their Original Properties.
From interactivesciencenotebook.weebly.com
Periodic Table & Chemical Reaction Notes Pratyusha's Science Notebook Do Elements Keep Or Lose Their Original Properties the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. the components of a mixture each keep their original properties. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. All of these elements display several other.. Do Elements Keep Or Lose Their Original Properties.
From www.slideserve.com
PPT Topic Chemistry Aim How are elements classified in the Do Elements Keep Or Lose Their Original Properties the components of a mixture each keep their original properties. All of these elements display several other. retention of individual properties: compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. the substances in a mixture also do not combine chemically to form a new substance, as. Do Elements Keep Or Lose Their Original Properties.
From www.alamy.com
Colorful Periodic Table of the Elements shows atomic number, symbol Do Elements Keep Or Lose Their Original Properties the elements in the periodic table are arranged in order of increasing atomic number. In a mixture, the components do not lose their individual properties. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. retention of individual properties: whereas a compound may have very. Do Elements Keep Or Lose Their Original Properties.
From www.wikihow.com
3 Ways to Study the Chemical and Physical Properties of Atoms in the Do Elements Keep Or Lose Their Original Properties whereas a compound may have very different properties from the elements that compose it, in mixtures the. the components of a mixture each keep their original properties. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. the elements in the periodic table are arranged. Do Elements Keep Or Lose Their Original Properties.
From www.periodictableprintable.com
Periodic Table Elements Lose Or Gain Electrons 2024 Periodic Table Do Elements Keep Or Lose Their Original Properties the elements in the periodic table are arranged in order of increasing atomic number. All of these elements display several other. whereas a compound may have very different properties from the elements that compose it, in mixtures the. the substances in a mixture also do not combine chemically to form a new substance, as they do in. Do Elements Keep Or Lose Their Original Properties.
From en.m.wikipedia.org
FilePeriodic table of elements showing electron shells.png Wikipedia Do Elements Keep Or Lose Their Original Properties the elements in the periodic table are arranged in order of increasing atomic number. In a mixture, the components do not lose their individual properties. the components of a mixture each keep their original properties. The separation of components can be easily done. All of these elements display several other. retention of individual properties: the substances. Do Elements Keep Or Lose Their Original Properties.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Do Elements Keep Or Lose Their Original Properties The separation of components can be easily done. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. In a mixture, the components do not lose their individual properties. All of these elements display several other. whereas a compound may have very different properties from the elements that compose. Do Elements Keep Or Lose Their Original Properties.
From removeandreplace.com
Periodic Table Of Elements With Names And Symbols Do Elements Keep Or Lose Their Original Properties discuss the relationships between matter, mass, elements, compounds, atoms, and. the components of a mixture each keep their original properties. The separation of components can be easily done. retention of individual properties: In a mixture, the components do not lose their individual properties. the substances in a mixture also do not combine chemically to form a. Do Elements Keep Or Lose Their Original Properties.
From data1.skinnyms.com
Printable Table Of Elements Do Elements Keep Or Lose Their Original Properties the components of a mixture each keep their original properties. whereas a compound may have very different properties from the elements that compose it, in mixtures the. All of these elements display several other. The separation of components can be easily done. the substances in a mixture also do not combine chemically to form a new substance,. Do Elements Keep Or Lose Their Original Properties.
From www.rd.com
Illustrated Periodic Table Shows How We Use Each Element Reader's Digest Do Elements Keep Or Lose Their Original Properties compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. The separation of components can be easily done. discuss the relationships between matter, mass, elements, compounds, atoms, and. whereas a compound may have very different properties from the elements that compose it, in mixtures the. the components. Do Elements Keep Or Lose Their Original Properties.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Do Elements Keep Or Lose Their Original Properties the components of a mixture each keep their original properties. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. All of these elements display several other. retention of individual properties: discuss the relationships between matter, mass, elements, compounds, atoms, and. the elements in. Do Elements Keep Or Lose Their Original Properties.
From glossary.periodni.com
Periodic table of the elements Chemistry Dictionary & Glossary Do Elements Keep Or Lose Their Original Properties retention of individual properties: In a mixture, the components do not lose their individual properties. whereas a compound may have very different properties from the elements that compose it, in mixtures the. The separation of components can be easily done. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent. Do Elements Keep Or Lose Their Original Properties.
From www.sciencelearn.org.nz
Periodic table of elements — Science Learning Hub Do Elements Keep Or Lose Their Original Properties the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. The separation of components can be easily done. discuss the relationships between matter, mass, elements, compounds, atoms, and. whereas a compound may have very different properties from the elements that compose it, in mixtures the. In. Do Elements Keep Or Lose Their Original Properties.
From topforeignstocks.com
Periodic Table Then and Now Infographics Do Elements Keep Or Lose Their Original Properties the components of a mixture each keep their original properties. All of these elements display several other. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. discuss the relationships between matter, mass, elements, compounds, atoms, and. compounds have specific chemical and physical properties that. Do Elements Keep Or Lose Their Original Properties.
From www.thoughtco.com
Properties of Periodic Table of Element Groups Do Elements Keep Or Lose Their Original Properties whereas a compound may have very different properties from the elements that compose it, in mixtures the. All of these elements display several other. the elements in the periodic table are arranged in order of increasing atomic number. The separation of components can be easily done. the substances in a mixture also do not combine chemically to. Do Elements Keep Or Lose Their Original Properties.
From projectopenletter.com
The Periodic Table List Of Elements Printable Form, Templates and Letter Do Elements Keep Or Lose Their Original Properties compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. whereas a compound may have very different properties from the elements that compose it, in mixtures the. The separation of components can be easily done. All of these elements display several other. the components of a mixture each. Do Elements Keep Or Lose Their Original Properties.
From capepac.org
Periodic Table Of Elements Names And Symbols List In Order Do Elements Keep Or Lose Their Original Properties In a mixture, the components do not lose their individual properties. the elements in the periodic table are arranged in order of increasing atomic number. retention of individual properties: the components of a mixture each keep their original properties. discuss the relationships between matter, mass, elements, compounds, atoms, and. whereas a compound may have very. Do Elements Keep Or Lose Their Original Properties.
From slideplayer.com
Compounds and Mixtures ppt video online download Do Elements Keep Or Lose Their Original Properties The separation of components can be easily done. discuss the relationships between matter, mass, elements, compounds, atoms, and. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. the elements in the periodic table are arranged in order of increasing atomic number. compounds have specific. Do Elements Keep Or Lose Their Original Properties.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Do Elements Keep Or Lose Their Original Properties retention of individual properties: whereas a compound may have very different properties from the elements that compose it, in mixtures the. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. In a mixture, the components do not lose their individual properties. All of these elements display several. Do Elements Keep Or Lose Their Original Properties.
From elchoroukhost.net
Periodic Table Of The Elements Definition Biology Elcho Table Do Elements Keep Or Lose Their Original Properties All of these elements display several other. the components of a mixture each keep their original properties. discuss the relationships between matter, mass, elements, compounds, atoms, and. The separation of components can be easily done. retention of individual properties: the substances in a mixture also do not combine chemically to form a new substance, as they. Do Elements Keep Or Lose Their Original Properties.
From dxojfykit.blob.core.windows.net
What Are Table Elements In at Roberta Cusick blog Do Elements Keep Or Lose Their Original Properties the elements in the periodic table are arranged in order of increasing atomic number. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. retention of individual properties: the components of a mixture each keep their original properties. The separation of components can be easily. Do Elements Keep Or Lose Their Original Properties.
From crackittoday.com
Einsteinium Properties Of Element 99 In The Periodic Table UPSC Notes Do Elements Keep Or Lose Their Original Properties retention of individual properties: discuss the relationships between matter, mass, elements, compounds, atoms, and. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. whereas a compound may have very different properties from the elements that compose it, in mixtures the. The separation of components. Do Elements Keep Or Lose Their Original Properties.
From sciencenotes.org
How to Use a Periodic Table Do Elements Keep Or Lose Their Original Properties The separation of components can be easily done. discuss the relationships between matter, mass, elements, compounds, atoms, and. In a mixture, the components do not lose their individual properties. All of these elements display several other. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. . Do Elements Keep Or Lose Their Original Properties.
From www.chemeurope.com
The Complete Periodic Table of Elements Do Elements Keep Or Lose Their Original Properties In a mixture, the components do not lose their individual properties. retention of individual properties: the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. the elements in the periodic table are arranged in order of increasing atomic number. All of these elements display several other.. Do Elements Keep Or Lose Their Original Properties.
From www.slideserve.com
PPT Physical Properties of Metals PowerPoint Presentation ID5519685 Do Elements Keep Or Lose Their Original Properties the components of a mixture each keep their original properties. discuss the relationships between matter, mass, elements, compounds, atoms, and. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. The separation of components can be easily done. All of these elements display several other. the substances. Do Elements Keep Or Lose Their Original Properties.
From bezybrowser.weebly.com
Periodic table simple states solid liquid gas bezybrowser Do Elements Keep Or Lose Their Original Properties In a mixture, the components do not lose their individual properties. the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. The separation of components can be easily. Do Elements Keep Or Lose Their Original Properties.
From brilliant.org
Periodic Table of the Elements Brilliant Math & Science Wiki Do Elements Keep Or Lose Their Original Properties the elements in the periodic table are arranged in order of increasing atomic number. discuss the relationships between matter, mass, elements, compounds, atoms, and. retention of individual properties: In a mixture, the components do not lose their individual properties. The separation of components can be easily done. compounds have specific chemical and physical properties that are. Do Elements Keep Or Lose Their Original Properties.
From period-faqs.com
How Does A Periodic Table Work Do Elements Keep Or Lose Their Original Properties the substances in a mixture also do not combine chemically to form a new substance, as they do in a compound. the elements in the periodic table are arranged in order of increasing atomic number. the components of a mixture each keep their original properties. compounds have specific chemical and physical properties that are distinct from. Do Elements Keep Or Lose Their Original Properties.
From periodictable.me
IUPAC Periodic Table of Elements with Name Dynamic Periodic Table of Do Elements Keep Or Lose Their Original Properties retention of individual properties: In a mixture, the components do not lose their individual properties. whereas a compound may have very different properties from the elements that compose it, in mixtures the. the components of a mixture each keep their original properties. the substances in a mixture also do not combine chemically to form a new. Do Elements Keep Or Lose Their Original Properties.
From sciencenotes.org
Periodic Table Blocks of Elements Do Elements Keep Or Lose Their Original Properties the components of a mixture each keep their original properties. retention of individual properties: The separation of components can be easily done. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. whereas a compound may have very different properties from the elements that compose it, in. Do Elements Keep Or Lose Their Original Properties.
From elchoroukhost.net
Periodic Table Of The Elements Definition Biology Elcho Table Do Elements Keep Or Lose Their Original Properties discuss the relationships between matter, mass, elements, compounds, atoms, and. the components of a mixture each keep their original properties. the elements in the periodic table are arranged in order of increasing atomic number. compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. retention of. Do Elements Keep Or Lose Their Original Properties.
From www.compoundchem.com
The Periodic Table of Elements Element Name Origins Compound Interest Do Elements Keep Or Lose Their Original Properties compounds have specific chemical and physical properties that are distinct from their constituent elements because the constituent elements lose. In a mixture, the components do not lose their individual properties. whereas a compound may have very different properties from the elements that compose it, in mixtures the. retention of individual properties: The separation of components can be. Do Elements Keep Or Lose Their Original Properties.
From www.ck12.org
The Periodic Table and Electron Configurations CK12 Foundation Do Elements Keep Or Lose Their Original Properties whereas a compound may have very different properties from the elements that compose it, in mixtures the. discuss the relationships between matter, mass, elements, compounds, atoms, and. the components of a mixture each keep their original properties. retention of individual properties: the substances in a mixture also do not combine chemically to form a new. Do Elements Keep Or Lose Their Original Properties.