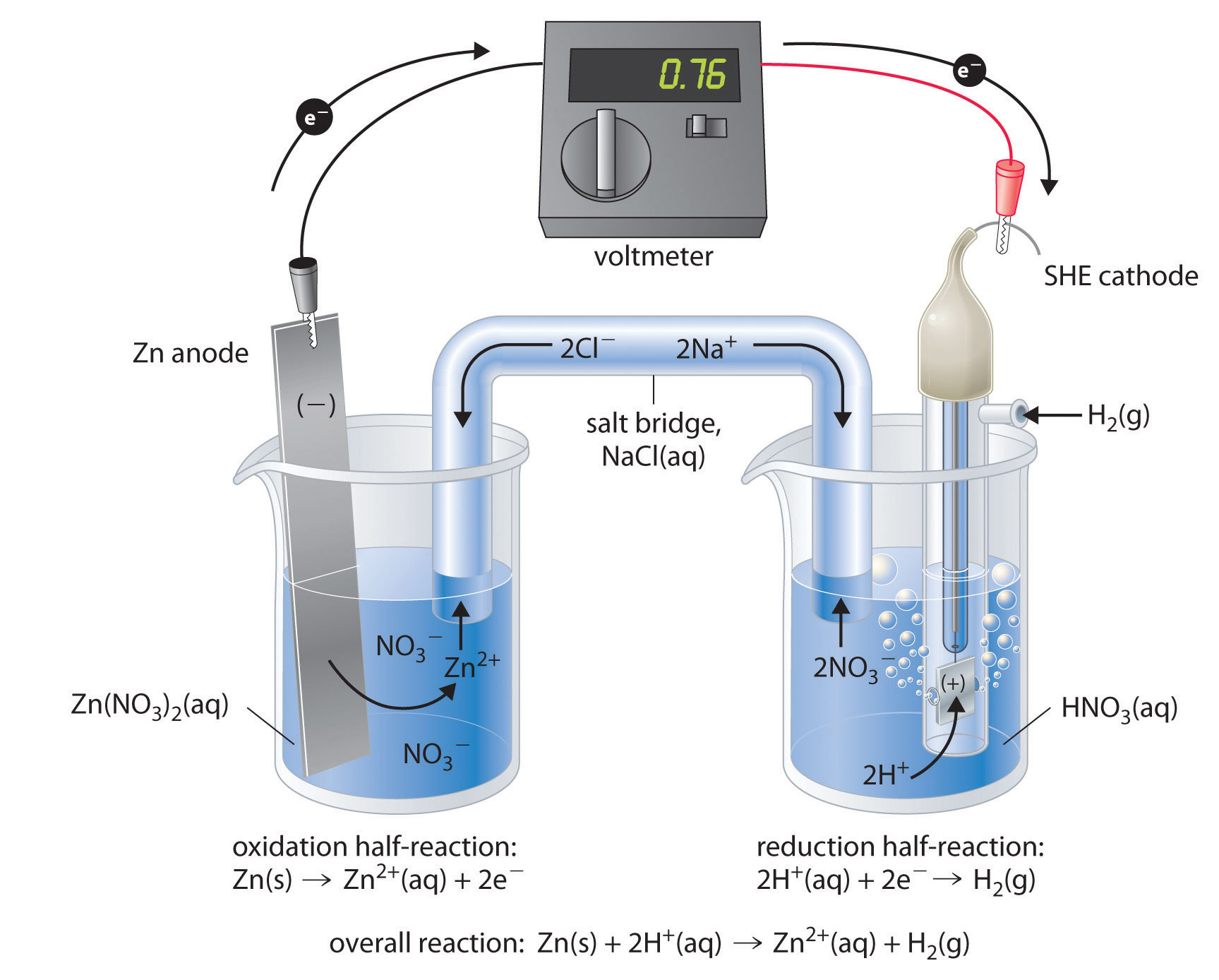
Standard Electrode Potential Vs Standard Reduction Potential . The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The potential of a half.
from saylordotorg.github.io
The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Describe and relate the definitions of electrode and cell potentials. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:.
Standard Potentials
Standard Electrode Potential Vs Standard Reduction Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:.
From www.nagwa.com
Question Video Calculating the Standard Reduction Potential of an Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of a half. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction. Standard Electrode Potential Vs Standard Reduction Potential.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the standard hydrogen electrode (she) is defined as 0 v under. Standard Electrode Potential Vs Standard Reduction Potential.
From www.pveducation.org
Standard Potential PVEducation Standard Electrode Potential Vs Standard Reduction Potential Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of a half. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is. Standard Electrode Potential Vs Standard Reduction Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential Vs Standard Reduction Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of the. Standard Electrode Potential Vs Standard Reduction Potential.
From www.nagwa.com
Question Video Identifying Conditions for Measuring Standard Electrode Standard Electrode Potential Vs Standard Reduction Potential Describe and relate the definitions of electrode and cell potentials. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of a half. The potential of the standard hydrogen electrode (she) is. Standard Electrode Potential Vs Standard Reduction Potential.
From vpia.org.vn
Standard Reduction Potentials made easy, reduction line Standard Electrode Potential Vs Standard Reduction Potential Describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of the standard hydrogen electrode (she) is defined. Standard Electrode Potential Vs Standard Reduction Potential.
From www.pinterest.com
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Electrode Potential Vs Standard Reduction Potential The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the standard hydrogen electrode (she) is. Standard Electrode Potential Vs Standard Reduction Potential.
From www.slideserve.com
PPT Oxidation and Reduction PowerPoint Presentation, free download Standard Electrode Potential Vs Standard Reduction Potential Describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard. Standard Electrode Potential Vs Standard Reduction Potential.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Electrode Potential Vs Standard Reduction Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of the standard hydrogen electrode (she) is defined as 0 v under. Standard Electrode Potential Vs Standard Reduction Potential.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Vs Standard Reduction Potential The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in. Standard Electrode Potential Vs Standard Reduction Potential.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Electrode Potential Vs Standard Reduction Potential The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the. Standard Electrode Potential Vs Standard Reduction Potential.
From saylordotorg.github.io
Standard Potentials Standard Electrode Potential Vs Standard Reduction Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. Interpret electrode potentials in terms of relative oxidant and reductant.. Standard Electrode Potential Vs Standard Reduction Potential.
From www.researchgate.net
Electrochemical scales in water (standard potentials of reduction vs Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode. Standard Electrode Potential Vs Standard Reduction Potential.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk Standard Electrode Potential Vs Standard Reduction Potential The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of the standard hydrogen electrode (she) is. Standard Electrode Potential Vs Standard Reduction Potential.
From www.researchgate.net
4 Potential relation between the experimentally applied Standard Standard Electrode Potential Vs Standard Reduction Potential The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to. Standard Electrode Potential Vs Standard Reduction Potential.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Vs Standard Reduction Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and. Standard Electrode Potential Vs Standard Reduction Potential.
From mungfali.com
Standard Electrode Potential Table Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard reduction. Standard Electrode Potential Vs Standard Reduction Potential.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Electrode Potential Vs Standard Reduction Potential Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard reduction potential is defined relative to the. Standard Electrode Potential Vs Standard Reduction Potential.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Electrode Potential Vs Standard Reduction Potential The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she),. Standard Electrode Potential Vs Standard Reduction Potential.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of a half. Describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and. Standard Electrode Potential Vs Standard Reduction Potential.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. Interpret electrode potentials in terms of relative oxidant. Standard Electrode Potential Vs Standard Reduction Potential.
From www.semanticscholar.org
[PDF] Evaluation of Ag/AgClelectrode standard potential uncertainty Standard Electrode Potential Vs Standard Reduction Potential The potential of a half. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard. Standard Electrode Potential Vs Standard Reduction Potential.
From www.youtube.com
19.1 Standard electrode potential (HL) YouTube Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. The potential of a. Standard Electrode Potential Vs Standard Reduction Potential.
From www.numerade.com
SOLVED Please help with the last two subquestions. THIS IS THE ONLY Standard Electrode Potential Vs Standard Reduction Potential The potential of a half. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. Describe and relate the definitions of electrode and cell potentials. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and. Standard Electrode Potential Vs Standard Reduction Potential.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Vs Standard Reduction Potential The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell. Standard Electrode Potential Vs Standard Reduction Potential.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Electrode Potential Vs Standard Reduction Potential The potential of a half. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Describe and relate the definitions of electrode and cell potentials. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the. Standard Electrode Potential Vs Standard Reduction Potential.
From facts.net
16 Unbelievable Facts About Standard Electrode Potential Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in. Standard Electrode Potential Vs Standard Reduction Potential.
From www.youtube.com
Difference between Oxidation potential and Reduction potential Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Describe and relate the definitions of electrode and cell potentials. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The potential of a half. The standard reduction potential is defined relative to the. Standard Electrode Potential Vs Standard Reduction Potential.
From www.researchgate.net
Standard reduction potential (V vs Ag/AgCl) of different elements in Standard Electrode Potential Vs Standard Reduction Potential The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of a half. Describe and relate the definitions. Standard Electrode Potential Vs Standard Reduction Potential.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. 372 rows the data below tabulates standard electrode. Standard Electrode Potential Vs Standard Reduction Potential.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Electrode Potential Vs Standard Reduction Potential The potential of a half. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials. Standard Electrode Potential Vs Standard Reduction Potential.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Electrode Potential Vs Standard Reduction Potential The potential of a half. Describe and relate the definitions of electrode and cell potentials. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she),. Standard Electrode Potential Vs Standard Reduction Potential.
From www.youtube.com
Standard Reduction Potential YouTube Standard Electrode Potential Vs Standard Reduction Potential 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Describe and relate the definitions of electrode and cell potentials. The potential of a half. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and. Standard Electrode Potential Vs Standard Reduction Potential.
From ablogwithadifference.com
Single Electrode Potential and Standard Electrode PotentialThe best 5 Standard Electrode Potential Vs Standard Reduction Potential The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The potential of a half. The standard reduction potential is defined relative to the standard hydrogen electrode (she). Standard Electrode Potential Vs Standard Reduction Potential.
From www.differencebetween.com
What is the Difference Between Standard Electrode Potential and Standard Electrode Potential Vs Standard Reduction Potential The potential of a half. Interpret electrode potentials in terms of relative oxidant and reductant. The standard reduction potential is defined relative to the standard hydrogen electrode (she) used as reference electrode, which is arbitrarily. Describe and relate the definitions of electrode and cell potentials. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard. Standard Electrode Potential Vs Standard Reduction Potential.