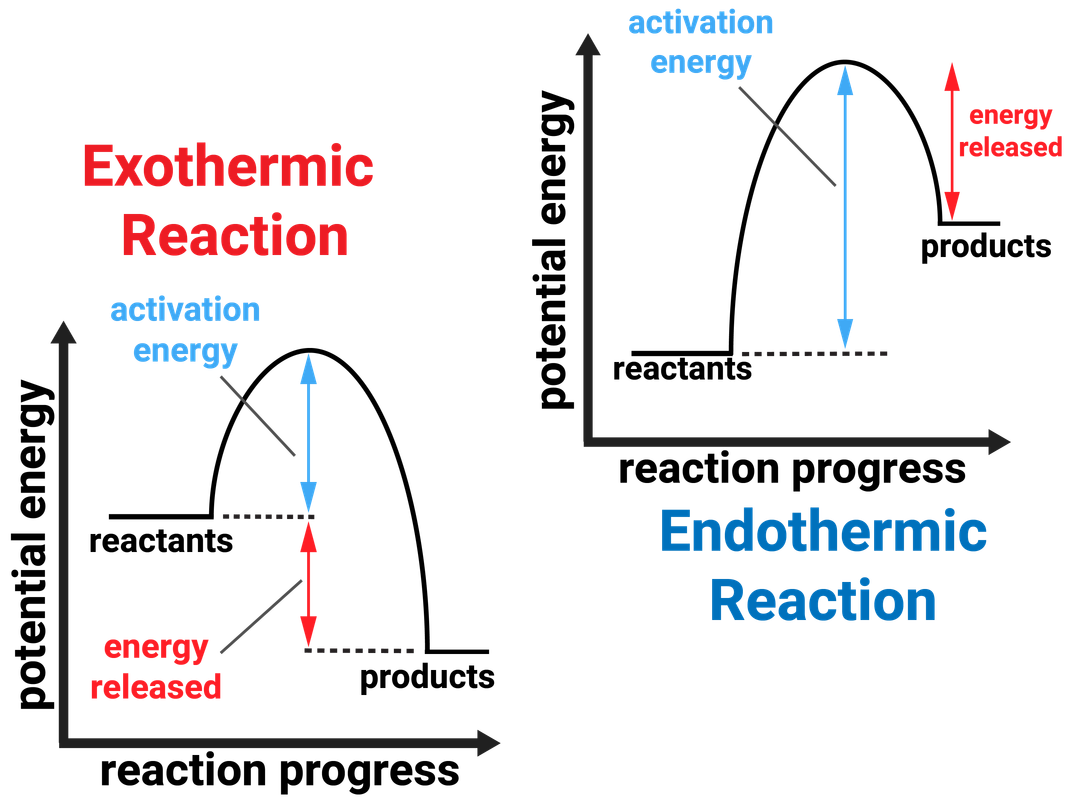
Endothermic Reaction Value Of Energy . Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. define endothermic and exothermic reactions. chemical reactions that release energy are called exothermic. therefore, δh is positive (δh>0). a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. From this positive change in enthalpy, one can tell if the reaction is endothermic or not. In exothermic reactions, more energy is released when the bonds are formed in the products.
from wiredatatisse56wr.z22.web.core.windows.net
define endothermic and exothermic reactions. chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products. therefore, δh is positive (δh>0). Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. From this positive change in enthalpy, one can tell if the reaction is endothermic or not.
Endothermic Reaction Energy Profile Diagram
Endothermic Reaction Value Of Energy define endothermic and exothermic reactions. chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. From this positive change in enthalpy, one can tell if the reaction is endothermic or not. define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. therefore, δh is positive (δh>0).
From byjus.com
24. What is the Minimum activation energy required for endothermic and exothermic reaction? Endothermic Reaction Value Of Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. Describe how heat is transferred in endothermic and exothermic. chemical reactions that release energy are. Endothermic Reaction Value Of Energy.
From kanayatichemistry.blogspot.com
What is an Endothermic Reaction in IGCSE CHEMISTRY ? K Chemistry Endothermic Reaction Value Of Energy a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. From this positive change in enthalpy, one can tell if the reaction is endothermic or not. In exothermic reactions, more energy is released when the bonds are formed in the products. learn about the energy. Endothermic Reaction Value Of Energy.
From materialmediabrindle.z21.web.core.windows.net
Endothermic Reactions And Exothermic Reaction Endothermic Reaction Value Of Energy learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that release energy are called exothermic. a chemical reaction is exothermic if the chemical energy. Endothermic Reaction Value Of Energy.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Value Of Energy From this positive change in enthalpy, one can tell if the reaction is endothermic or not. therefore, δh is positive (δh>0). Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. In exothermic reactions, more energy is released when the bonds are formed in the products. in endothermic and exothermic reactions, energy can. Endothermic Reaction Value Of Energy.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction Value Of Energy In exothermic reactions, more energy is released when the bonds are formed in the products. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. therefore, δh is positive (δh>0). Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. learn about the. Endothermic Reaction Value Of Energy.
From www.slideserve.com
PPT Potential Energy Diagrams PowerPoint Presentation, free download ID442094 Endothermic Reaction Value Of Energy a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. learn about the energy of chemical bonds in compounds and explain how energy values are. Endothermic Reaction Value Of Energy.
From app.pandai.org
Energy Changes in Chemical Reactions Endothermic Reaction Value Of Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that release energy are called exothermic. . Endothermic Reaction Value Of Energy.
From www.tes.com
Exothermic and endothermic temperature changes, energy change graphs, and bonds. Complete Lesson Endothermic Reaction Value Of Energy In exothermic reactions, more energy is released when the bonds are formed in the products. Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. a chemical reaction is exothermic if the chemical energy. Endothermic Reaction Value Of Energy.
From www.doubtnut.com
For an endothermic reaction, where Delta H represents the enthalpy of Endothermic Reaction Value Of Energy chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products. therefore, δh is positive (δh>0). Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. . Endothermic Reaction Value Of Energy.
From wiredatatisse56wr.z22.web.core.windows.net
Endothermic Reaction Energy Profile Diagram Endothermic Reaction Value Of Energy a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that release energy are called exothermic. in endothermic and exothermic reactions, energy can be thought of as. Endothermic Reaction Value Of Energy.
From stock.adobe.com
Vecteur Stock Vector graphs or charts of endothermic and exothermic reactions physics Endothermic Reaction Value Of Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. In exothermic reactions, more energy is released when the bonds are formed in the products. Describe how heat is transferred in endothermic and exothermic. learn about the energy of chemical bonds in compounds and explain how energy values are. Endothermic Reaction Value Of Energy.
From www.sarthaks.com
For an endothermic reaction, where `Delta H` represents the enthalpy of reaction in `kJ mol^(1 Endothermic Reaction Value Of Energy learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. define endothermic and exothermic reactions. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. In exothermic reactions, more energy is released when. Endothermic Reaction Value Of Energy.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Value Of Energy chemical reactions that release energy are called exothermic. therefore, δh is positive (δh>0). learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or. Endothermic Reaction Value Of Energy.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Value Of Energy From this positive change in enthalpy, one can tell if the reaction is endothermic or not. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that release. Endothermic Reaction Value Of Energy.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Value Of Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. learn about the energy of chemical bonds in compounds and explain how energy values are. Endothermic Reaction Value Of Energy.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Coordinate Diagram Endothermic Reaction Value Of Energy a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. therefore, δh is positive (δh>0). define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic. learn about the energy of chemical bonds in compounds and explain how energy values. Endothermic Reaction Value Of Energy.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID431456 Endothermic Reaction Value Of Energy chemical reactions that release energy are called exothermic. define endothermic and exothermic reactions. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting. Endothermic Reaction Value Of Energy.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Value Of Energy Describe how heat is transferred in endothermic and exothermic. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. chemical reactions that release energy are called exothermic. define endothermic and exothermic reactions. therefore, δh is positive (δh>0). From this positive change in enthalpy,. Endothermic Reaction Value Of Energy.
From schematicdiagramyakuza.z13.web.core.windows.net
Enthalpy Profile Diagram Endothermic Reaction Endothermic Reaction Value Of Energy chemical reactions that release energy are called exothermic. Describe how heat is transferred in endothermic and exothermic. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. a chemical reaction is exothermic if the chemical energy of products is lower than that. Endothermic Reaction Value Of Energy.
From slideplayer.com
Endothermic Vs. Exothermic Reaction Graphs ppt download Endothermic Reaction Value Of Energy therefore, δh is positive (δh>0). In exothermic reactions, more energy is released when the bonds are formed in the products. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. in endothermic and exothermic reactions, energy can be thought of as either. Endothermic Reaction Value Of Energy.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Value Of Energy in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. In exothermic reactions, more energy is released when the bonds are formed in the products. From this positive change in enthalpy, one can tell if the reaction is endothermic or not. define endothermic and exothermic reactions. learn about. Endothermic Reaction Value Of Energy.
From slideplayer.com
Endothermic and exothermic reactions ppt download Endothermic Reaction Value Of Energy define endothermic and exothermic reactions. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. in endothermic and. Endothermic Reaction Value Of Energy.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Value Of Energy therefore, δh is positive (δh>0). learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. define endothermic and exothermic reactions. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. chemical. Endothermic Reaction Value Of Energy.
From stock.adobe.com
activation energy endothermic reaction diagram vector de Stock Adobe Stock Endothermic Reaction Value Of Energy chemical reactions that release energy are called exothermic. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. In exothermic reactions, more energy is released. Endothermic Reaction Value Of Energy.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Value Of Energy a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that release energy are called exothermic. learn about the energy of chemical bonds in compounds and explain. Endothermic Reaction Value Of Energy.
From kunduz.com
[ANSWERED] For an endothermic reaction energy of activation is E and Kunduz Endothermic Reaction Value Of Energy In exothermic reactions, more energy is released when the bonds are formed in the products. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. chemical reactions that release energy are called exothermic. define endothermic and exothermic reactions. learn about the energy of. Endothermic Reaction Value Of Energy.
From guideelsk5f.z4.web.core.windows.net
Energy Diagram For Endothermic Reaction Endothermic Reaction Value Of Energy therefore, δh is positive (δh>0). Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a. Endothermic Reaction Value Of Energy.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Value Of Energy From this positive change in enthalpy, one can tell if the reaction is endothermic or not. In exothermic reactions, more energy is released when the bonds are formed in the products. in endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. a chemical reaction is exothermic if the chemical. Endothermic Reaction Value Of Energy.
From lessonschoolallodial.z13.web.core.windows.net
Endothermic And Exothermic Reaction Diagram Endothermic Reaction Value Of Energy Describe how heat is transferred in endothermic and exothermic. define endothermic and exothermic reactions. chemical reactions that release energy are called exothermic. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. learn about the energy of chemical bonds in compounds and explain. Endothermic Reaction Value Of Energy.
From asenoveo6schematic.z14.web.core.windows.net
How To Read Energy Diagrams Chemistry Endothermic Reaction Value Of Energy therefore, δh is positive (δh>0). From this positive change in enthalpy, one can tell if the reaction is endothermic or not. In exothermic reactions, more energy is released when the bonds are formed in the products. Describe how heat is transferred in endothermic and exothermic. in endothermic and exothermic reactions, energy can be thought of as either a. Endothermic Reaction Value Of Energy.
From www.slideserve.com
PPT Energy Changes PowerPoint Presentation, free download ID3470966 Endothermic Reaction Value Of Energy define endothermic and exothermic reactions. therefore, δh is positive (δh>0). learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. chemical reactions that release energy are called exothermic. Describe how heat is transferred in endothermic and exothermic. in endothermic and. Endothermic Reaction Value Of Energy.
From www.vedantu.com
For an endothermic reaction where {\\bf{\\Delta H}} represents the enthalpy of the reaction in Endothermic Reaction Value Of Energy learn about the energy of chemical bonds in compounds and explain how energy values are used to work out the energy change of a reaction. a chemical reaction is exothermic if the chemical energy of products is lower than that of the starting reactants, while an endothermic reaction. In exothermic reactions, more energy is released when the bonds. Endothermic Reaction Value Of Energy.
From chemnotcheem.com
Enthalpy change of reactions O Level Chemistry Notes Endothermic Reaction Value Of Energy From this positive change in enthalpy, one can tell if the reaction is endothermic or not. In exothermic reactions, more energy is released when the bonds are formed in the products. chemical reactions that release energy are called exothermic. learn about the energy of chemical bonds in compounds and explain how energy values are used to work out. Endothermic Reaction Value Of Energy.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Endothermic Reaction Value Of Energy From this positive change in enthalpy, one can tell if the reaction is endothermic or not. therefore, δh is positive (δh>0). chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products. Describe how heat is transferred in endothermic and exothermic. a chemical reaction is. Endothermic Reaction Value Of Energy.
From www.dreamstime.com
Activation Energy Endothermic Reaction 2d Illustration Stock Illustration Illustration of Endothermic Reaction Value Of Energy define endothermic and exothermic reactions. In exothermic reactions, more energy is released when the bonds are formed in the products. therefore, δh is positive (δh>0). chemical reactions that release energy are called exothermic. From this positive change in enthalpy, one can tell if the reaction is endothermic or not. Describe how heat is transferred in endothermic and. Endothermic Reaction Value Of Energy.