Do Bases Change The Color Of Indicators . A variety of indicators change color at various ph levels. In this article, the basics of. A ph value is determined from the negative. Acid and base indicators are molecules which change color in different ph environments. This makes them useful for. They are incredibly useful for quickly identifying the ph of a solution. Indicators are substances whose solutions change color due to changes in ph. Acids cause universal indicator solution to change from green. They are usually weak acids or. This is a charge of common. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its.
from chem.libretexts.org
Acid and base indicators are molecules which change color in different ph environments. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Acids cause universal indicator solution to change from green. They are incredibly useful for quickly identifying the ph of a solution. This is a charge of common. They are usually weak acids or. Indicators are substances whose solutions change color due to changes in ph. This makes them useful for. In this article, the basics of. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution.
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts
Do Bases Change The Color Of Indicators Acids cause universal indicator solution to change from green. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. This makes them useful for. This is a charge of common. Acid and base indicators are molecules which change color in different ph environments. A variety of indicators change color at various ph levels. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. They are usually weak acids or. In this article, the basics of. A ph value is determined from the negative. They are incredibly useful for quickly identifying the ph of a solution. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Indicators are substances whose solutions change color due to changes in ph. Acids cause universal indicator solution to change from green.
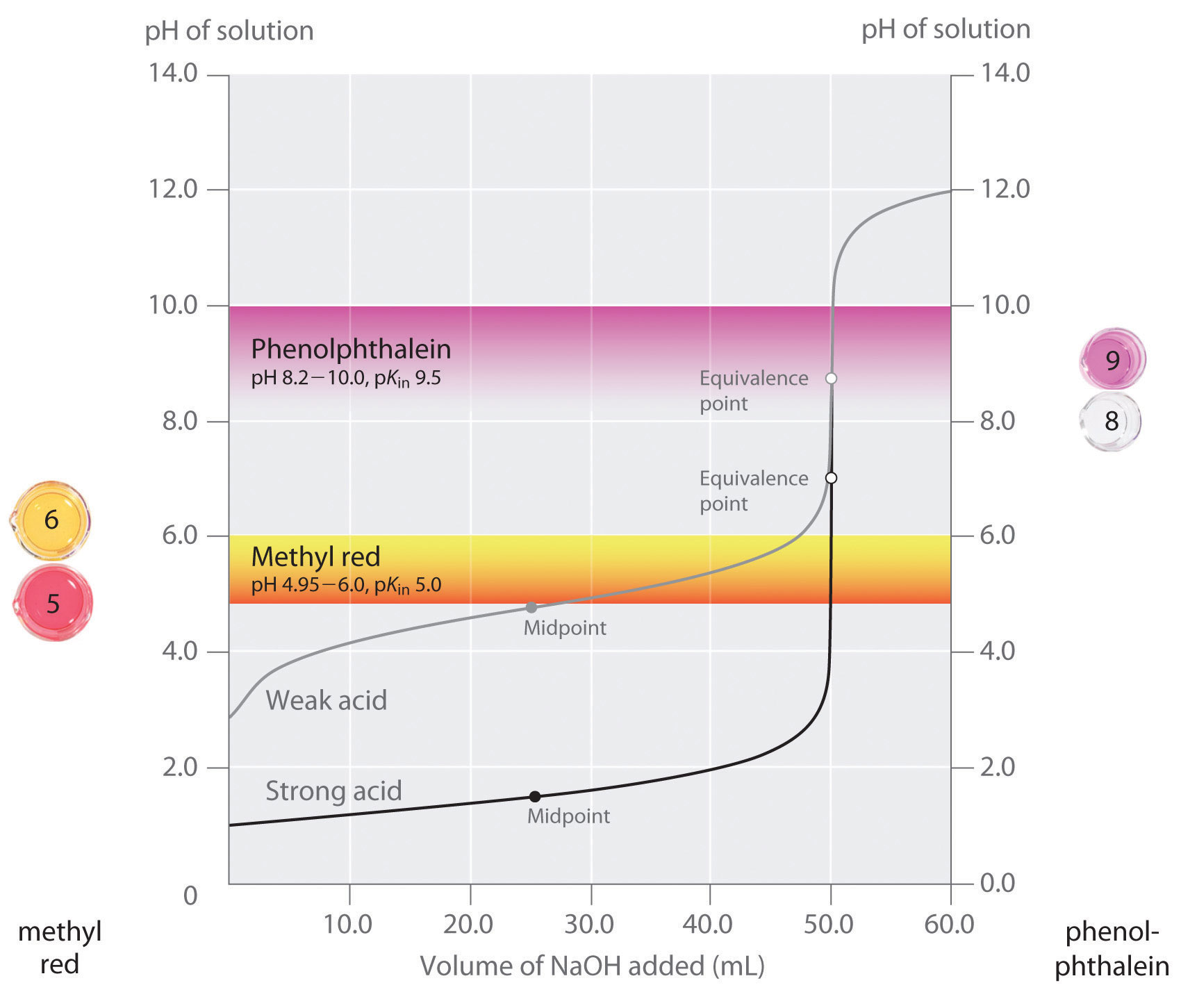
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ Do Bases Change The Color Of Indicators In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. They are incredibly useful for quickly identifying the ph of a solution. A variety of indicators change color at various ph levels. Acid and base indicators are molecules which change color. Do Bases Change The Color Of Indicators.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID5741622 Do Bases Change The Color Of Indicators This makes them useful for. They are usually weak acids or. Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. When universal indicator is added to a solution,. Do Bases Change The Color Of Indicators.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Do Bases Change The Color Of Indicators In this article, the basics of. This makes them useful for. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its. Do Bases Change The Color Of Indicators.
From chimactiv.agroparistech.fr
Chimactiv Interactive numerical educational resources for the Do Bases Change The Color Of Indicators Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Acids cause universal indicator solution to change from green. This makes them useful for. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Indicators. Do Bases Change The Color Of Indicators.
From www.youtube.com
AE3 Colour changes of indicators YouTube Do Bases Change The Color Of Indicators When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. They are incredibly useful for quickly identifying the ph of a solution. This is a charge of common. Acids cause universal indicator solution to change from green. A variety of indicators change color at various ph levels. A ph value is. Do Bases Change The Color Of Indicators.
From slideplayer.com
Acids, Bases and Salts. ppt download Do Bases Change The Color Of Indicators Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. This is a charge of common. A ph value is determined from the negative. They are incredibly useful for quickly identifying the ph of a solution. This makes them useful for. A variety of. Do Bases Change The Color Of Indicators.
From mavink.com
Acid And Base Indicators Do Bases Change The Color Of Indicators Indicators are substances whose solutions change color due to changes in ph. They are incredibly useful for quickly identifying the ph of a solution. In this article, the basics of. A ph value is determined from the negative. A variety of indicators change color at various ph levels. In an acidic solution, the colour indicator will exist in its conjugate. Do Bases Change The Color Of Indicators.
From smallbusinessron.web.fc2.com
what color does phenolphthalein turn in a base Do Bases Change The Color Of Indicators In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. This makes them useful for. In this article, the basics of. They are usually weak acids or. A variety of indicators change color at various ph levels. A ph value is. Do Bases Change The Color Of Indicators.
From mungfali.com
Acid Base Indicator Chart Do Bases Change The Color Of Indicators This is a charge of common. This makes them useful for. Acid and base indicators are molecules which change color in different ph environments. In this article, the basics of. Acids cause universal indicator solution to change from green. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added. Do Bases Change The Color Of Indicators.
From in.pinterest.com
𝐩𝐇 𝐢𝐧𝐝𝐢𝐜𝐚𝐭𝐨𝐫 𝐜𝐨𝐥𝐨𝐫𝐬 Organic chemistry books, Bromothymol blue Do Bases Change The Color Of Indicators In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. This is a charge of common. In this article, the basics of. They are usually weak acids or. Indicators are substances whose solutions change color due to changes in ph. Acids. Do Bases Change The Color Of Indicators.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Do Bases Change The Color Of Indicators Acid and base indicators are molecules which change color in different ph environments. A ph value is determined from the negative. They are usually weak acids or. Indicators are substances whose solutions change color due to changes in ph. They are incredibly useful for quickly identifying the ph of a solution. In an acidic solution, the colour indicator will exist. Do Bases Change The Color Of Indicators.
From www.science-sparks.com
How to make a red cabbage pH indicator Chemistry for Kids Do Bases Change The Color Of Indicators Acid and base indicators are molecules which change color in different ph environments. Acids cause universal indicator solution to change from green. In this article, the basics of. A variety of indicators change color at various ph levels. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and. Do Bases Change The Color Of Indicators.
From www.dreamstime.com
PH Indicator. Color Change of Blue Litmus Paper To Red for Acids, Red Do Bases Change The Color Of Indicators When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Acid and base indicators are molecules which change color in different ph environments. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Indicators are. Do Bases Change The Color Of Indicators.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Do Bases Change The Color Of Indicators This makes them useful for. A ph value is determined from the negative. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. When universal indicator is added to a solution, the color change can indicate the approximate ph of the. Do Bases Change The Color Of Indicators.
From www.slideshare.net
P h indicators Do Bases Change The Color Of Indicators A variety of indicators change color at various ph levels. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. They are usually weak acids or. This is a charge of common. In an acidic solution, the colour indicator will exist in its conjugate. Do Bases Change The Color Of Indicators.
From www.flinnsci.com
Universal Indicator Color Charts Do Bases Change The Color Of Indicators They are usually weak acids or. Acids cause universal indicator solution to change from green. Indicators are substances whose solutions change color due to changes in ph. They are incredibly useful for quickly identifying the ph of a solution. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added. Do Bases Change The Color Of Indicators.
From www.compoundchem.com
Compound Interest The Colours & Chemistry of pH Indicators Do Bases Change The Color Of Indicators Acids cause universal indicator solution to change from green. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Indicators are substances. Do Bases Change The Color Of Indicators.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Do Bases Change The Color Of Indicators Acids cause universal indicator solution to change from green. This makes them useful for. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. A ph value is determined from the negative. This is a charge of common. Indicators are substances whose solutions change color due to changes in ph. They. Do Bases Change The Color Of Indicators.
From www.slideserve.com
PPT AcidBase Theories PowerPoint Presentation, free download ID Do Bases Change The Color Of Indicators A ph value is determined from the negative. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Acid and base indicators are molecules which change color in different ph environments. Acids cause universal indicator solution to change from green. In an acidic solution, the colour indicator will exist in its. Do Bases Change The Color Of Indicators.
From www.chemistry4students.com
Chemistry 4 Students Acid Base indicators Do Bases Change The Color Of Indicators They are incredibly useful for quickly identifying the ph of a solution. Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. In this article, the basics of. They. Do Bases Change The Color Of Indicators.
From slideplayer.com
Acids, Bases & Salts. ppt download Do Bases Change The Color Of Indicators In this article, the basics of. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Indicators are substances whose solutions change color due to changes in ph. They are incredibly useful for quickly identifying the ph of a solution. Ph indicators are weak acids that exist as natural dyes and. Do Bases Change The Color Of Indicators.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Do Bases Change The Color Of Indicators Acid and base indicators are molecules which change color in different ph environments. They are usually weak acids or. This is a charge of common. Acids cause universal indicator solution to change from green. A variety of indicators change color at various ph levels. When universal indicator is added to a solution, the color change can indicate the approximate ph. Do Bases Change The Color Of Indicators.
From www.slideserve.com
PPT PROPERTIES OF ACIDS & BASES PowerPoint Presentation, free Do Bases Change The Color Of Indicators In this article, the basics of. This is a charge of common. A ph value is determined from the negative. Acids cause universal indicator solution to change from green. They are incredibly useful for quickly identifying the ph of a solution. They are usually weak acids or. This makes them useful for. Ph indicators are weak acids that exist as. Do Bases Change The Color Of Indicators.
From www.slideserve.com
PPT Acids, Bases, and pH PowerPoint Presentation, free download ID Do Bases Change The Color Of Indicators Indicators are substances whose solutions change color due to changes in ph. Acid and base indicators are molecules which change color in different ph environments. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. A variety of indicators change color at various ph levels. They are incredibly useful for quickly. Do Bases Change The Color Of Indicators.
From markettay.com
Tabla de indicadores de pH Colores y rangos Market tay Do Bases Change The Color Of Indicators In this article, the basics of. Indicators are substances whose solutions change color due to changes in ph. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. They are usually weak acids or. A variety of indicators change color at various ph levels. A ph value is determined from the. Do Bases Change The Color Of Indicators.
From davidswart.wordpress.com
Acids and Bases The Art of Science Do Bases Change The Color Of Indicators This makes them useful for. In this article, the basics of. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. This is a charge of common. Indicators are substances whose solutions change color due to changes in ph. Acids cause universal indicator solution to change from green. They are incredibly. Do Bases Change The Color Of Indicators.
From knowledge.carolina.com
AcidBase Indicators Carolina Knowledge Center Do Bases Change The Color Of Indicators Acids cause universal indicator solution to change from green. They are usually weak acids or. This makes them useful for. They are incredibly useful for quickly identifying the ph of a solution. A variety of indicators change color at various ph levels. This is a charge of common. Indicators are substances whose solutions change color due to changes in ph.. Do Bases Change The Color Of Indicators.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Do Bases Change The Color Of Indicators They are usually weak acids or. Acid and base indicators are molecules which change color in different ph environments. Indicators are substances whose solutions change color due to changes in ph. They are incredibly useful for quickly identifying the ph of a solution. Acids cause universal indicator solution to change from green. When universal indicator is added to a solution,. Do Bases Change The Color Of Indicators.
From slideplayer.com
Notes Science SPI Acids and Bases ppt download Do Bases Change The Color Of Indicators This makes them useful for. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Acid and base indicators are molecules which change color in different ph environments. Ph indicators are weak acids. Do Bases Change The Color Of Indicators.
From mavink.com
Acid Base Indicator Colors Do Bases Change The Color Of Indicators A variety of indicators change color at various ph levels. Acids cause universal indicator solution to change from green. Indicators are substances whose solutions change color due to changes in ph. This makes them useful for. They are incredibly useful for quickly identifying the ph of a solution. Ph indicators are weak acids that exist as natural dyes and indicate. Do Bases Change The Color Of Indicators.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Do Bases Change The Color Of Indicators They are incredibly useful for quickly identifying the ph of a solution. Indicators are substances whose solutions change color due to changes in ph. A ph value is determined from the negative. Acids cause universal indicator solution to change from green. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base. Do Bases Change The Color Of Indicators.
From www.slideserve.com
PPT Acids, Bases and Alkalis PowerPoint Presentation ID3063336 Do Bases Change The Color Of Indicators Acids cause universal indicator solution to change from green. Acid and base indicators are molecules which change color in different ph environments. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. A ph value is determined from the negative. Ph indicators are weak acids that exist as natural dyes and. Do Bases Change The Color Of Indicators.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Do Bases Change The Color Of Indicators Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. Acids cause universal indicator solution to change from green. In this article, the basics of. This is a charge of common. This makes them useful for. A variety of indicators change color at various. Do Bases Change The Color Of Indicators.
From guitarscalechart.z28.web.core.windows.net
universal indicator scale chart Ph scale to colour in Do Bases Change The Color Of Indicators They are usually weak acids or. When universal indicator is added to a solution, the color change can indicate the approximate ph of the solution. Acids cause universal indicator solution to change from green. A ph value is determined from the negative. Acid and base indicators are molecules which change color in different ph environments. This makes them useful for.. Do Bases Change The Color Of Indicators.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Do Bases Change The Color Of Indicators A variety of indicators change color at various ph levels. A ph value is determined from the negative. This is a charge of common. This makes them useful for. Ph indicators are weak acids that exist as natural dyes and indicate the concentration of h + (\ (h_3o^+\)) ions in a solution via color change. They are incredibly useful for. Do Bases Change The Color Of Indicators.