How Many Formula Units Are In 325 Grams Of Iron Ii Chloride . » fecl2 mass concentration to molar concentration converter. Determine the molar mass of iron (iii) chloride: 1.204 × 10^24 formula units. Using the following formula to convert mass of iron (iii) chloride to moles; We assume you are converting between grams iron (ii) chloride and mole. Let us start by calculating the formula mass of sodium chloride (nacl). Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. How many grams iron (ii) chloride in 1 mol? Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio.
from lab.honeywell.com
Using the following formula to convert mass of iron (iii) chloride to moles; We assume you are converting between grams iron (ii) chloride and mole. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. Determine the molar mass of iron (iii) chloride: Let us start by calculating the formula mass of sodium chloride (nacl). How many grams iron (ii) chloride in 1 mol? This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. 1.204 × 10^24 formula units. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. » fecl2 mass concentration to molar concentration converter.
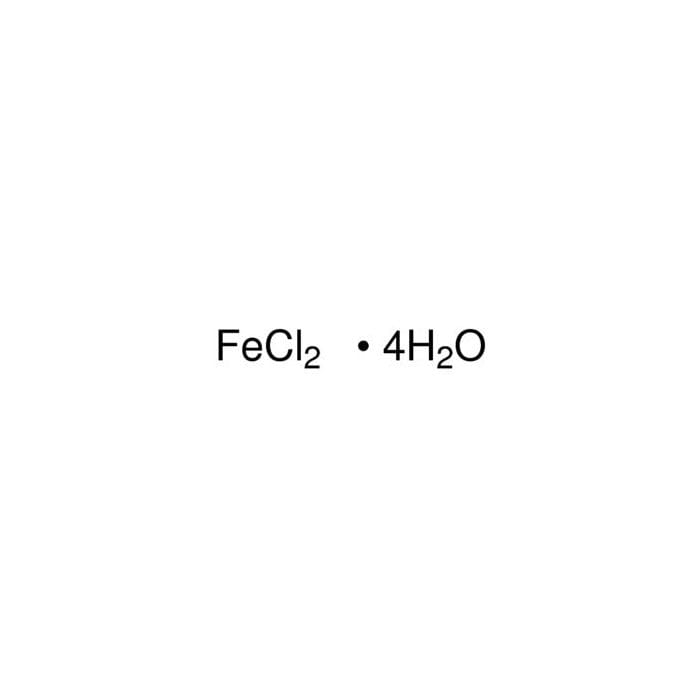
Iron(II) chloride tetrahydrate 44939 Honeywell Research Chemicals
How Many Formula Units Are In 325 Grams Of Iron Ii Chloride We assume you are converting between grams iron (ii) chloride and mole. Using the following formula to convert mass of iron (iii) chloride to moles; This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. 1.204 × 10^24 formula units. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. How many grams iron (ii) chloride in 1 mol? Determine the molar mass of iron (iii) chloride: We assume you are converting between grams iron (ii) chloride and mole. » fecl2 mass concentration to molar concentration converter. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. Let us start by calculating the formula mass of sodium chloride (nacl).
From www.fishersci.se
Iron(II) chloride, 97, anhydrous, Thermo Scientific Chemicals Fisher How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Let us start by calculating the formula mass of sodium chloride (nacl). 1.204 × 10^24 formula units. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. We assume you are converting between grams iron (ii) chloride and mole. Determine the molar mass of iron (iii) chloride: How many grams. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.flinnsci.com
Iron(II) Chloride, Reagent, 100 g Flinn Scientific How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. Determine the molar mass of iron (iii) chloride: Let us start by calculating the formula mass of sodium chloride (nacl). » fecl2 mass concentration to molar concentration converter. Using the following formula to convert mass of iron (iii). How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.numerade.com
SOLVED Iron reacts with hydrochloric acid to produce iron(II) chloride How Many Formula Units Are In 325 Grams Of Iron Ii Chloride 1.204 × 10^24 formula units. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. Let us start by calculating the formula mass of sodium chloride (nacl). How many grams iron (ii) chloride in 1 mol? » fecl2 mass concentration to molar concentration converter. Fecl3 = 1 iron. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.youtube.com
Lewis Structure of Iron (II) Chloride, FeCl2 YouTube How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Let us start by calculating the formula mass of sodium chloride (nacl). Determine the molar mass of iron (iii) chloride: Using the following formula to convert mass of iron (iii) chloride to moles; We assume you are converting between grams iron (ii) chloride and mole. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions,. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.fishersci.ca
Iron(II) chloride tetrahydrate, 98, Thermo Scientific Chemicals How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Determine the molar mass of iron (iii) chloride: Let us start by calculating the formula mass of sodium chloride (nacl). Using the following formula to convert mass of iron (iii) chloride to moles; This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. We assume you are converting between grams. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.numerade.com
SOLVED How many formula units of sodium chloride are in 14.6 grams of How Many Formula Units Are In 325 Grams Of Iron Ii Chloride We assume you are converting between grams iron (ii) chloride and mole. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. » fecl2 mass concentration to molar. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.chegg.com
Solved How many formula units of iron (II) nitride are in How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Let us start by calculating the formula mass of sodium chloride (nacl). Determine the molar mass of iron (iii) chloride: This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. 1.204 × 10^24 formula units. » fecl2 mass concentration to molar concentration converter. Using the following formula to convert mass. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.flinnsci.com
Flinn Chemicals, Iron(II) Chloride How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. We assume you are converting between grams iron (ii) chloride and mole. Determine the molar mass of. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.sciencephoto.com
Iron (II) chloride crystals Stock Image A710/0061 Science Photo How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Determine the molar mass of iron (iii) chloride: 1.204 × 10^24 formula units. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. How many grams iron (ii) chloride in 1 mol? Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From lab.honeywell.com
Iron(II) chloride tetrahydrate 44939 Honeywell Research Chemicals How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Let us start by calculating the formula mass of sodium chloride (nacl). This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. How many grams iron (ii) chloride in. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slidesharetrick.blogspot.com
Iron Ii Chloride Formula slidesharetrick How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. How many grams iron (ii) chloride in 1 mol? Let us start by calculating the formula mass of sodium chloride (nacl). » fecl2 mass concentration to molar concentration converter. Using the following formula to convert mass of iron (iii). How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slidesharetrick.blogspot.com
Iron Ii Chloride Formula slidesharetrick How Many Formula Units Are In 325 Grams Of Iron Ii Chloride » fecl2 mass concentration to molar concentration converter. Determine the molar mass of iron (iii) chloride: We assume you are converting between grams iron (ii) chloride and mole. Using the following formula to convert mass of iron (iii) chloride to moles; How many grams iron (ii) chloride in 1 mol? Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.slideserve.com
PPT Prentice Hall Chemistry (c) 2005 PowerPoint Presentation, free How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Determine the molar mass of iron (iii) chloride: We assume you are converting between grams iron (ii) chloride and mole. How many grams iron (ii) chloride in 1 mol? 1.204 × 10^24 formula units. Let us start by calculating the formula mass of sodium chloride (nacl). Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From jkenterprises.com.pk
Iron(II) chloride tetrahydrate, 99+ J K Enterprises Chemical J K How Many Formula Units Are In 325 Grams Of Iron Ii Chloride How many grams iron (ii) chloride in 1 mol? Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. Determine the molar mass of iron (iii) chloride: » fecl2. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.numerade.com
SOLVED Stoichiometry (Mass Mass) Reaction Use question4 2 Al (s) 3 How Many Formula Units Are In 325 Grams Of Iron Ii Chloride We assume you are converting between grams iron (ii) chloride and mole. How many grams iron (ii) chloride in 1 mol? Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. 1.204 × 10^24 formula units. Determine the molar mass of iron (iii) chloride: This formula mass is the. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slideplayer.com
Chemical Names and Formulas ppt download How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Using the following formula to convert mass of iron (iii) chloride to moles; Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. » fecl2 mass concentration to molar concentration converter. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom,. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From general.chemistrysteps.com
How To Convert Grams To Moles Chemistry Steps How Many Formula Units Are In 325 Grams Of Iron Ii Chloride How many grams iron (ii) chloride in 1 mol? Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. » fecl2 mass concentration to molar concentration converter. We assume you are converting between grams iron (ii) chloride and mole. 1.204 × 10^24 formula units. Using the following formula to. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.sciencephoto.com
Iron chlorides Stock Image C011/4551 Science Photo Library How Many Formula Units Are In 325 Grams Of Iron Ii Chloride 1.204 × 10^24 formula units. Let us start by calculating the formula mass of sodium chloride (nacl). Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. How many grams iron (ii) chloride in 1 mol? This formula mass is the sum of the atomic masses of one sodium. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.difference.wiki
Iron II Chloride vs. Iron III Chloride What’s the Difference? How Many Formula Units Are In 325 Grams Of Iron Ii Chloride 1.204 × 10^24 formula units. How many grams iron (ii) chloride in 1 mol? Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. » fecl2 mass concentration to molar concentration converter. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −,. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slideplayer.com
Classifying Chemical Compounds ppt download How Many Formula Units Are In 325 Grams Of Iron Ii Chloride How many grams iron (ii) chloride in 1 mol? 1.204 × 10^24 formula units. We assume you are converting between grams iron (ii) chloride and mole. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. Determine the molar mass of iron (iii) chloride: Sodium chloride is an ionic compound. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.numerade.com
SOLVED How many formula units make up 36.4 g of magnesium chloride How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Determine the molar mass of iron (iii) chloride: Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. 1.204 × 10^24 formula units. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. Using the following formula. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.slideserve.com
PPT Review of Atomic Model PowerPoint Presentation, free download How Many Formula Units Are In 325 Grams Of Iron Ii Chloride This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. Let us start by calculating the formula mass of sodium chloride (nacl). Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. Fecl3 = 1 iron atom (55.85. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.numerade.com
SOLVED PLEASE HELP ME!! CHEMISTRY 139. How many formula units are How Many Formula Units Are In 325 Grams Of Iron Ii Chloride » fecl2 mass concentration to molar concentration converter. We assume you are converting between grams iron (ii) chloride and mole. How many grams iron (ii) chloride in 1 mol? Let us start by calculating the formula mass of sodium chloride (nacl). Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.wikidoc.org
Ferrous chloride wikidoc How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Using the following formula to convert mass of iron (iii) chloride to moles; How many grams iron (ii) chloride in 1 mol? 1.204 × 10^24 formula units. Let us start by calculating the formula mass of sodium chloride (nacl). Determine the molar mass of iron (iii) chloride: Sodium chloride is an ionic compound composed of sodium cations, na +, and. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.sciencephoto.com
Iron (II) chloride solution Stock Image C029/5906 Science Photo How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Using the following formula to convert mass of iron (iii) chloride to moles; This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. How many grams iron (ii) chloride in 1 mol? Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.chegg.com
Solved 13. When iron (II) chloride reacts with potassium How Many Formula Units Are In 325 Grams Of Iron Ii Chloride How many grams iron (ii) chloride in 1 mol? Using the following formula to convert mass of iron (iii) chloride to moles; » fecl2 mass concentration to molar concentration converter. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. Fecl3 = 1 iron atom (55.85 g/mol) +. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.numerade.com
SOLVED According to the following reaction, how many grams of iron(II How Many Formula Units Are In 325 Grams Of Iron Ii Chloride » fecl2 mass concentration to molar concentration converter. Using the following formula to convert mass of iron (iii) chloride to moles; 1.204 × 10^24 formula units. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. We assume you are converting between grams iron (ii) chloride and mole. Sodium. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From alchetron.com
Iron(II) chloride Alchetron, The Free Social Encyclopedia How Many Formula Units Are In 325 Grams Of Iron Ii Chloride This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. » fecl2 mass concentration to molar concentration converter. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. Sodium chloride is an ionic compound composed of sodium cations, na. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slidesharetrick.blogspot.com
Iron Ii Chloride Formula slidesharetrick How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. 1.204 × 10^24 formula units. This formula mass is the sum of the atomic masses of one. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slideplayer.com
Ionic Compounds Involving Transition Metals ppt download How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Determine the molar mass of iron (iii) chloride: Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. 1.204 × 10^24 formula units. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. We assume you are converting. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slideplayer.com
The Mole. ppt download How Many Formula Units Are In 325 Grams Of Iron Ii Chloride » fecl2 mass concentration to molar concentration converter. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. 1.204 × 10^24 formula units. Determine the molar mass of. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slideplayer.com
IONIC COMPOUNDS WITH Multivalent Ions ppt download How Many Formula Units Are In 325 Grams Of Iron Ii Chloride We assume you are converting between grams iron (ii) chloride and mole. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. Determine the molar mass of iron (iii). How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.chegg.com
Solved 1. In 0.45 mol of Iron(III) chloride 2. How many How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Using the following formula to convert mass of iron (iii) chloride to moles; 1.204 × 10^24 formula units. » fecl2 mass concentration to molar concentration converter. Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. We assume you are converting between grams iron (ii) chloride and mole. How. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From slideplayer.com
Writing Chemical Formulas. ppt download How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Determine the molar mass of iron (iii) chloride: Sodium chloride is an ionic compound composed of sodium cations, na +, and chloride anions, cl −, combined in a 1:1 ratio. » fecl2 mass concentration to molar concentration converter. This formula mass is the sum of the atomic masses of one sodium atom and one chlorine atom, which we. How many. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.
From www.youtube.com
How to Write the Formula for Iron (II) chlorate YouTube How Many Formula Units Are In 325 Grams Of Iron Ii Chloride Determine the molar mass of iron (iii) chloride: How many grams iron (ii) chloride in 1 mol? Fecl3 = 1 iron atom (55.85 g/mol) + 3 chlorine atoms (3 x 35.45 g/mol) = 162.2 g/mol step 2/3 2. Let us start by calculating the formula mass of sodium chloride (nacl). » fecl2 mass concentration to molar concentration converter. Sodium chloride. How Many Formula Units Are In 325 Grams Of Iron Ii Chloride.