Low Ionic Strength Buffer . (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Let “i” equal ionic strength of the solution. Use this formula to calculate ionic strength: These commonly observed problems can be attributed to the. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. Purified water that has very few ionic species is said to be. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. The formula in step 1 states that ionic. Determines the buffer ion concentrations but also depends. They significantly reduce the stabilisation time required and increase. From eqn [1], the following properties of a buffer solution can be easily derived:
from www.slideserve.com
A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. They significantly reduce the stabilisation time required and increase. The formula in step 1 states that ionic. Determines the buffer ion concentrations but also depends. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Use this formula to calculate ionic strength: Purified water that has very few ionic species is said to be. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. From eqn [1], the following properties of a buffer solution can be easily derived: The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples.
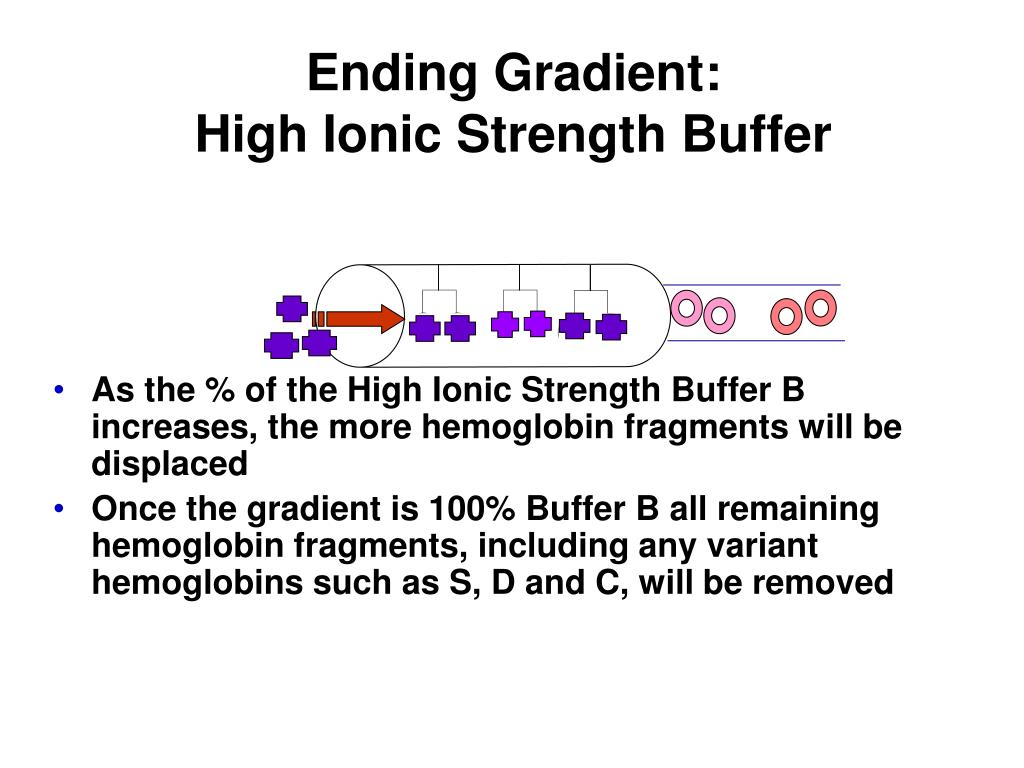
PPT Hemoglobin Electrophoresis PowerPoint Presentation, free download
Low Ionic Strength Buffer A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. Use this formula to calculate ionic strength: From eqn [1], the following properties of a buffer solution can be easily derived: Let “i” equal ionic strength of the solution. Purified water that has very few ionic species is said to be. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. The formula in step 1 states that ionic. Determines the buffer ion concentrations but also depends. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. They significantly reduce the stabilisation time required and increase. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. These commonly observed problems can be attributed to the.
From www.researchgate.net
Activity of T4 lysozyme in low ionic strength buffer. M. luteus Low Ionic Strength Buffer Determines the buffer ion concentrations but also depends. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. The formula in step 1 states that ionic. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. From. Low Ionic Strength Buffer.
From www.vacutaineradditives.com
Low Ionic Strength Hygroscopic Tromethamine Tris Buffer Low Ionic Strength Buffer (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. Purified water that has very few ionic species is said to be. The ph of high purity water is generally in the. Low Ionic Strength Buffer.
From www.jbc.org
Intranasal M Cell Uptake of Nanoparticles Is Independently Influenced Low Ionic Strength Buffer The formula in step 1 states that ionic. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. These commonly observed problems can be attributed to the. From eqn [1], the following properties of a buffer solution can be easily derived: (1) at low ionic strength (i.e., i. Low Ionic Strength Buffer.
From www.researchgate.net
Low ionic strength buffers supporting specific hybridization. Using the Low Ionic Strength Buffer These commonly observed problems can be attributed to the. Determines the buffer ion concentrations but also depends. The formula in step 1 states that ionic. They significantly reduce the stabilisation time required and increase. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. From eqn [1], the. Low Ionic Strength Buffer.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Low Ionic Strength Buffer Determines the buffer ion concentrations but also depends. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. They significantly reduce the stabilisation time required and increase. From eqn [1], the following properties of a buffer solution can be easily derived: The standard calibration with ph buffer of ph. Low Ionic Strength Buffer.
From www.researchgate.net
A schematic of a calcite surface in low and high ionic strength Low Ionic Strength Buffer (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Let “i” equal ionic strength of the solution. They significantly reduce the stabilisation time required and increase. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. The formula in step 1 states. Low Ionic Strength Buffer.
From www.researchgate.net
Initial composition and ionic strengths of the Donnan pore water in the Low Ionic Strength Buffer Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. From eqn [1], the following properties of a buffer solution can be easily derived: The formula in. Low Ionic Strength Buffer.
From cehypoid.blob.core.windows.net
Meaning Of Low Ionic Strength at Robert Moody blog Low Ionic Strength Buffer Determines the buffer ion concentrations but also depends. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. A series of special buffers which are suitable for accurate measurement of low ionic. Low Ionic Strength Buffer.
From www.researchgate.net
The effect of the ionic strength of a mixture of various buffers on the Low Ionic Strength Buffer A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. They significantly reduce the stabilisation time required and increase. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. From eqn [1], the following properties of a buffer solution can be easily derived:. Low Ionic Strength Buffer.
From answers.seneye.com
Effect of ionic strength on PH tests Seneye Low Ionic Strength Buffer A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Use. Low Ionic Strength Buffer.
From cymitquimica.com
Reagecon pH 6.96 Low Ionic Strength Buffer Solution at 20°C 07LS695 Low Ionic Strength Buffer The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. They significantly reduce the stabilisation time required and increase. Use this formula to calculate ionic strength: From eqn [1], the following properties of a buffer solution can be easily derived: The formula in step 1 states that ionic.. Low Ionic Strength Buffer.
From www.researchgate.net
Subviral components of IPNV. Visualization by EM of filamentous Low Ionic Strength Buffer The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. Determines the buffer ion concentrations but also depends. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. They significantly reduce. Low Ionic Strength Buffer.
From www.spex.com
SPEX CertiPrep SPEX CertiPrep ISBUF2500 10M Sodium Hydroxide (NaOH Low Ionic Strength Buffer The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. They significantly reduce the stabilisation time required and increase. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. The formula. Low Ionic Strength Buffer.
From www.camlab.co.uk
Low Ionic Strength Buffers pH Values At 20°C Low Ionic Strength Buffer The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. Let “i” equal ionic strength of the solution. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. Use this formula. Low Ionic Strength Buffer.
From www.researchgate.net
Fig. S5 Ionic Strength of the buffer (left axis) and added NaCl Low Ionic Strength Buffer The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. From eqn [1], the following properties of a buffer solution can be easily derived: Purified water that has very few ionic species is said to be. Starting with a given ph, the buffer ion concentrations can be calculated. Low Ionic Strength Buffer.
From www.slideserve.com
PPT Hemoglobin Electrophoresis PowerPoint Presentation, free download Low Ionic Strength Buffer These commonly observed problems can be attributed to the. Determines the buffer ion concentrations but also depends. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. From eqn [1], the following properties of a buffer solution can be easily derived: Starting with. Low Ionic Strength Buffer.
From www.researchgate.net
Comparison of the amounts of tropomyosin, tropomodulin and actin Low Ionic Strength Buffer Use this formula to calculate ionic strength: The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. Determines the buffer ion concentrations but also depends. They significantly reduce the stabilisation time required and increase. (1) at low ionic strength (i.e., i → 0. Low Ionic Strength Buffer.
From www.researchgate.net
Fig. S5 Ionic Strength of the buffer (left axis) and added NaCl Low Ionic Strength Buffer Purified water that has very few ionic species is said to be. They significantly reduce the stabilisation time required and increase. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. The standard calibration with ph buffer of ph 4.01 and ph 7.00. Low Ionic Strength Buffer.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Low Ionic Strength Buffer Use this formula to calculate ionic strength: From eqn [1], the following properties of a buffer solution can be easily derived: Determines the buffer ion concentrations but also depends. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. The standard calibration with. Low Ionic Strength Buffer.
From www.researchgate.net
Rheological assessment of bulk properties of keratin polymers. All Low Ionic Strength Buffer The formula in step 1 states that ionic. Purified water that has very few ionic species is said to be. From eqn [1], the following properties of a buffer solution can be easily derived: (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. These commonly observed problems can be attributed to the. The. Low Ionic Strength Buffer.
From www.amazon.com
Total Ionic Strength Adjustment Buffer (TISAB II), with CDTA for Low Ionic Strength Buffer These commonly observed problems can be attributed to the. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Let “i” equal ionic strength of the solution. From eqn [1], the following properties. Low Ionic Strength Buffer.
From cehypoid.blob.core.windows.net
Meaning Of Low Ionic Strength at Robert Moody blog Low Ionic Strength Buffer Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. Determines the buffer ion concentrations but also depends. Purified water that has very few ionic species is said to be. The formula in step 1 states that ionic. They significantly reduce the stabilisation time required and increase. Let. Low Ionic Strength Buffer.
From www.slideserve.com
PPT Hemoglobin Electrophoresis PowerPoint Presentation ID6656725 Low Ionic Strength Buffer (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Use this formula to calculate ionic strength: The formula in step 1 states that ionic. Purified water that has very few ionic species is said to be. They significantly reduce the stabilisation time required and increase. The standard calibration with ph buffer of ph. Low Ionic Strength Buffer.
From www.researchgate.net
(PDF) Measurement of Low Avidity, Polyreactive Immunoglobulin G Low Ionic Strength Buffer These commonly observed problems can be attributed to the. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. The formula in step 1 states that ionic. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. The ph of high purity. Low Ionic Strength Buffer.
From aslopubs.onlinelibrary.wiley.com
Measuring pH in low ionic strength glacial meltwaters using ion Low Ionic Strength Buffer The formula in step 1 states that ionic. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. Determines the buffer ion concentrations but also depends. From eqn [1], the following properties of a buffer solution can be easily derived: (1) at low ionic strength (i.e., i →. Low Ionic Strength Buffer.
From www.researchgate.net
(a) Ionic strength dependence of the 2D PC hydrogel in CHES buffer (pH Low Ionic Strength Buffer The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. Use this formula to calculate ionic strength: (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Determines the buffer ion concentrations but also depends. The standard. Low Ionic Strength Buffer.
From www.researchgate.net
Calibration curve of the ISFET in drops of buffer with low ionic Low Ionic Strength Buffer The formula in step 1 states that ionic. From eqn [1], the following properties of a buffer solution can be easily derived: Purified water that has very few ionic species is said to be. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. The standard calibration with. Low Ionic Strength Buffer.
From www.researchgate.net
Ion exchange chromatography of HeLa histones. Low ionic strength Low Ionic Strength Buffer Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. Use this formula to calculate ionic strength: Determines the buffer ion. Low Ionic Strength Buffer.
From www.researchgate.net
Formation of GUVs using buffers with different ionic strengths Low Ionic Strength Buffer These commonly observed problems can be attributed to the. Use this formula to calculate ionic strength: A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. (1). Low Ionic Strength Buffer.
From www.researchgate.net
8 A temporal change in morphology of DNA condensates is not correlated Low Ionic Strength Buffer Purified water that has very few ionic species is said to be. Use this formula to calculate ionic strength: The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water samples. Determines the buffer ion concentrations but also depends. Let “i” equal ionic strength of the solution. These commonly observed. Low Ionic Strength Buffer.
From www.researchgate.net
Effect of (a) pH, (b) ionic strength of the buffer, (c) voltage, and Low Ionic Strength Buffer The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. Purified water that has very few ionic species is said to be. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to measure water. Low Ionic Strength Buffer.
From www.revvity.com
HTRF PPI Low Ionic Strength Europium Detection buffer, 220 mL Low Ionic Strength Buffer From eqn [1], the following properties of a buffer solution can be easily derived: The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide (co 2) in the water. The standard calibration with ph buffer of ph 4.01 and ph 7.00 (@25°c) is sufficient in most cases to. Low Ionic Strength Buffer.
From www.researchgate.net
The effect of the ionic strength of a mixture of various buffers on the Low Ionic Strength Buffer (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. Starting with a given ph, the buffer ion concentrations can be calculated including temperature effects and the ionic strength of the solution. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. From. Low Ionic Strength Buffer.
From www.researchgate.net
Scheme of the ion's dissociation and hydration in buffer solution with Low Ionic Strength Buffer Purified water that has very few ionic species is said to be. (1) at low ionic strength (i.e., i → 0 and γ → 1), the solution shows. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. The formula in step 1 states that ionic. Starting with a. Low Ionic Strength Buffer.
From www.sentek.co.uk
Ionic Strength Adjustment Buffer (ISAB) for ISEs Sentek Limited Low Ionic Strength Buffer Let “i” equal ionic strength of the solution. These commonly observed problems can be attributed to the. A series of special buffers which are suitable for accurate measurement of low ionic strength samples when used with standard electrodes. The ph of high purity water is generally in the range of 5.5 to 7.5, depending on the level of carbon dioxide. Low Ionic Strength Buffer.