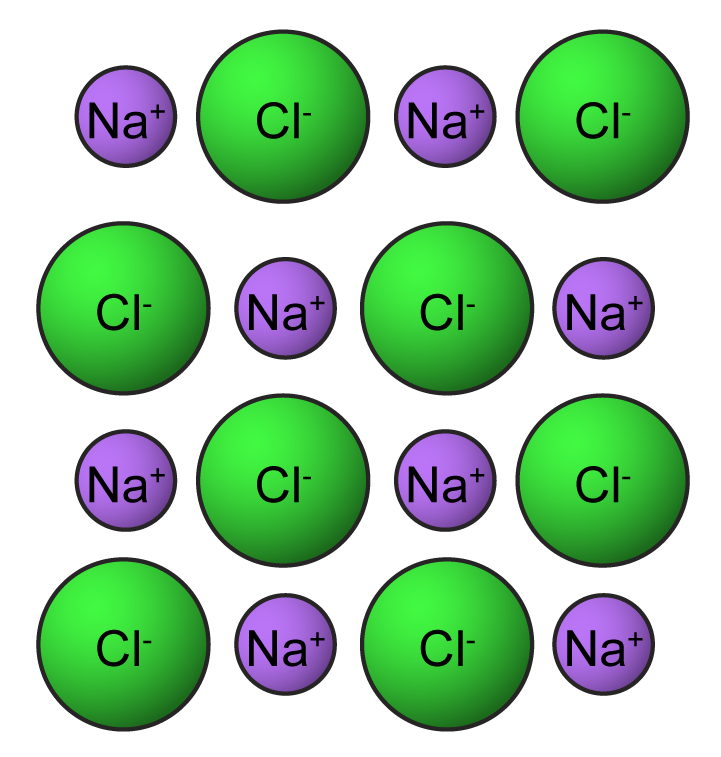
Brittle Ionic Compounds . Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. They conduct electricity when they are dissolved in water. ionic compounds are hard and brittle. summary of ionic compound properties. Ionic compounds are generally hard and brittle. See the study guide on. Ionic compounds dissociate into ions when dissolved in water. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. ionic compounds are generally hard, but brittle. your starting assumption that ionic compounds are frequently brittle is misleading. here are the properties of ionic compounds: Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. It takes a large amount of mechanical force, such as striking a crystal with a. Many, if not most, solids are. Ionic compounds generally have high.
from paperwingrvice.web.fc2.com
ionic compounds are generally hard, but brittle. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. Ionic compounds are generally hard and brittle. See the study guide on. Ionic compounds share several similar properties: ionic compounds are hard and brittle. High melting and boiling points;. your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a. Ionic compounds dissociate into ions when dissolved in water.
Why are ionic compounds brittle?
Brittle Ionic Compounds the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. They conduct electricity when they are dissolved in water. here are the properties of ionic compounds: Ionic compounds dissociate into ions when dissolved in water. Many, if not most, solids are. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compounds are generally hard, but brittle. Ionic compounds generally have high. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are generally hard and brittle. your starting assumption that ionic compounds are frequently brittle is misleading. summary of ionic compound properties. Ionic compounds share several similar properties: It takes a large amount of mechanical force, such as striking a crystal with a. See the study guide on.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Brittle Ionic Compounds Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. ionic compounds are hard and brittle. here are the properties of ionic compounds: your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a.. Brittle Ionic Compounds.
From www.wikidoc.org
Ionic compound wikidoc Brittle Ionic Compounds here are the properties of ionic compounds: summary of ionic compound properties. ionic compounds are generally hard, but brittle. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. High melting and boiling points;. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not.. Brittle Ionic Compounds.
From www.slideserve.com
PPT MYP Chemistry Ionic Bonding and Ionic Compounds PowerPoint Brittle Ionic Compounds ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. here are the properties of ionic compounds: your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds. Brittle Ionic Compounds.
From thechemistrynotes.com
Ionic Lattice and Ionic Compounds Brittle Ionic Compounds Many, if not most, solids are. your starting assumption that ionic compounds are frequently brittle is misleading. ionic compounds are hard and brittle. They conduct electricity when they are dissolved in water. here are the properties of ionic compounds: High melting and boiling points;. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials. Brittle Ionic Compounds.
From paperwingrvice.web.fc2.com
Why are ionic compounds brittle? Brittle Ionic Compounds Ionic compounds share several similar properties: See the study guide on. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. High melting and boiling points;. Ionic compounds are generally hard and brittle. ionic compounds are hard and brittle. Many, if not most, solids are. Ionic compounds have high melting. Brittle Ionic Compounds.
From paperwingrvice.web.fc2.com
Why are ionic compounds brittle? Brittle Ionic Compounds Ionic compounds are generally hard and brittle. ionic compounds are generally hard, but brittle. High melting and boiling points;. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. summary of ionic compound properties. Ionic compounds dissociate into ions when dissolved in water. They conduct electricity when they are dissolved in water. Ionic. Brittle Ionic Compounds.
From www.slideserve.com
PPT Properties of Ionic, Covalent, and Metallic Compounds PowerPoint Brittle Ionic Compounds ionic compounds are generally hard, but brittle. Ionic compounds dissociate into ions when dissolved in water. See the study guide on. Ionic compounds share several similar properties: here are the properties of ionic compounds: High melting and boiling points;. ionic compounds are hard and brittle. the electrostatic repulsion can be enough to split the crystal, which. Brittle Ionic Compounds.
From www.studocu.com
Covalent Compounds Worksheet Ionic compounds are brittle, covalent Brittle Ionic Compounds Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Many, if not most, solids are. See the study guide on. They conduct electricity when they are dissolved in water. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. summary of ionic compound properties.. Brittle Ionic Compounds.
From www.ck12.org
Ionic Compounds CK12 Foundation Brittle Ionic Compounds They conduct electricity when they are dissolved in water. It takes a large amount of mechanical force, such as striking a crystal with a. your starting assumption that ionic compounds are frequently brittle is misleading. summary of ionic compound properties. High melting and boiling points;. ionic compounds are hard and brittle. See the study guide on. . Brittle Ionic Compounds.
From slideplayer.com
Chemical Compounds Chapter 10 Pages ppt download Brittle Ionic Compounds Ionic compounds share several similar properties: Many, if not most, solids are. It takes a large amount of mechanical force, such as striking a crystal with a. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. See the study guide on. here are the properties of ionic compounds: Ionic. Brittle Ionic Compounds.
From slideplayer.com
Chapter 5 The Structure of Matter ppt download Brittle Ionic Compounds your starting assumption that ionic compounds are frequently brittle is misleading. They conduct electricity when they are dissolved in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are generally hard and brittle. Ionic compounds share several similar properties: ionic compounds are hard and brittle. Ionic. Brittle Ionic Compounds.
From www.doubtnut.com
Explain why ionic compounds are hard and brittle? Brittle Ionic Compounds Ionic compounds dissociate into ions when dissolved in water. ionic compounds are hard and brittle. Many, if not most, solids are. your starting assumption that ionic compounds are frequently brittle is misleading. It takes a large amount of mechanical force, such as striking a crystal with a. Ionic compounds have high melting and boiling points, so they are. Brittle Ionic Compounds.
From nftpanel.net
How Do Ionic Bonds Affect the Properties of Ionic Compounds Brittle Ionic Compounds Ionic compounds share several similar properties: It takes a large amount of mechanical force, such as striking a crystal with a. Ionic compounds are generally hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. summary of ionic compound properties. Ionic compounds dissociate into ions when dissolved in water. the. Brittle Ionic Compounds.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Brittle Ionic Compounds Ionic compounds dissociate into ions when dissolved in water. Ionic compounds share several similar properties: It takes a large amount of mechanical force, such as striking a crystal with a. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. They conduct electricity when they are dissolved in water. summary of ionic compound properties.. Brittle Ionic Compounds.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5980343 Brittle Ionic Compounds Many, if not most, solids are. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are generally hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds dissociate into ions when dissolved in water. They conduct electricity when they. Brittle Ionic Compounds.
From www.youtube.com
Why ionic solids are brittle? YouTube Brittle Ionic Compounds High melting and boiling points;. See the study guide on. here are the properties of ionic compounds: summary of ionic compound properties. They conduct electricity when they are dissolved in water. your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds have high melting and boiling points, so they are in the solid state. Brittle Ionic Compounds.
From www.chemistry-teaching-resources.com
chemistry picture Brittle Ionic Compounds ionic compounds are hard and brittle. They conduct electricity when they are dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds dissociate into ions when dissolved in water. summary of ionic compound properties. High melting and boiling points;. Ionic compounds share several similar properties: See the study. Brittle Ionic Compounds.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Brittle Ionic Compounds here are the properties of ionic compounds: Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. summary of ionic compound properties. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. They conduct electricity when they are dissolved in water.. Brittle Ionic Compounds.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Brittle Ionic Compounds your starting assumption that ionic compounds are frequently brittle is misleading. High melting and boiling points;. Ionic compounds dissociate into ions when dissolved in water. ionic compounds are generally hard, but brittle. It takes a large amount of mechanical force, such as striking a crystal with a. Ionic compounds are generally hard and brittle. They conduct electricity when. Brittle Ionic Compounds.
From slideplayer.com
Ionic Bonding Lesson ppt download Brittle Ionic Compounds Ionic compounds are generally hard and brittle. ionic compounds are generally hard, but brittle. here are the properties of ionic compounds: Ionic compounds share several similar properties: It takes a large amount of mechanical force, such as striking a crystal with a. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids. Brittle Ionic Compounds.
From www.youtube.com
Why are ionic compounds hard and brittle? YouTube Brittle Ionic Compounds ionic compounds are hard and brittle. Many, if not most, solids are. See the study guide on. Ionic compounds dissociate into ions when dissolved in water. ionic compounds are generally hard, but brittle. Ionic compounds share several similar properties: Ionic compounds generally have high. It takes a large amount of mechanical force, such as striking a crystal with. Brittle Ionic Compounds.
From chem.libretexts.org
2.1 Chemical Compounds Chemistry LibreTexts Brittle Ionic Compounds Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. High melting and boiling points;. ionic compounds are hard and brittle. here are the properties of ionic compounds: summary of ionic compound properties. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. . Brittle Ionic Compounds.
From slideplayer.com
Ionic Bonding. ppt download Brittle Ionic Compounds your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds share several similar properties: Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds are generally hard and brittle. Ionic compounds dissociate into ions when dissolved in water. ionic compounds are generally hard, but brittle.. Brittle Ionic Compounds.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Brittle Ionic Compounds Ionic compounds share several similar properties: ionic compounds are hard and brittle. ionic compounds are generally hard, but brittle. Many, if not most, solids are. here are the properties of ionic compounds: the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. It takes a large amount of. Brittle Ionic Compounds.
From slideplayer.com
Chapter 20 Section ppt download Brittle Ionic Compounds Ionic compounds share several similar properties: Ionic compounds dissociate into ions when dissolved in water. Ionic compounds generally have high. Many, if not most, solids are. High melting and boiling points;. They conduct electricity when they are dissolved in water. summary of ionic compound properties. It takes a large amount of mechanical force, such as striking a crystal with. Brittle Ionic Compounds.
From slideplayer.com
Structure and Properties of Bonds ppt download Brittle Ionic Compounds summary of ionic compound properties. here are the properties of ionic compounds: Ionic compounds share several similar properties: Ionic compounds generally have high. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Ionic compounds dissociate into ions when dissolved in water. They conduct electricity when they are dissolved in. Brittle Ionic Compounds.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Brittle Ionic Compounds ionic compounds are hard and brittle. Ionic compounds are generally hard and brittle. High melting and boiling points;. It takes a large amount of mechanical force, such as striking a crystal with a. here are the properties of ionic compounds: ionic compounds are generally hard, but brittle. Many, if not most, solids are. Ionic compounds share several. Brittle Ionic Compounds.
From getchemistry.weebly.com
Ionic Bonding Chemistry Brittle Ionic Compounds Ionic compounds dissociate into ions when dissolved in water. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. ionic compounds are generally hard, but brittle. Ionic compounds are generally hard and brittle. See the study guide on. Ionic compounds generally have high. Ionic compounds have high melting and boiling. Brittle Ionic Compounds.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Brittle Ionic Compounds They conduct electricity when they are dissolved in water. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds generally have high. summary of ionic compound properties. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. ionic compounds are generally hard,. Brittle Ionic Compounds.
From www.numerade.com
SOLVED (c) K forms the compound KzO,which is an ionic compound that is Brittle Ionic Compounds here are the properties of ionic compounds: Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. See the study guide on. ionic compounds are generally hard, but brittle. ionic. Brittle Ionic Compounds.
From mungfali.com
What Are Ionic Compounds Brittle Ionic Compounds Ionic compounds are generally hard and brittle. See the study guide on. here are the properties of ionic compounds: Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. summary of ionic compound properties. They conduct electricity when they are dissolved in water. Ionic compounds share several similar properties: Ionic compounds generally have. Brittle Ionic Compounds.
From slideplayer.com
Ionic Bonding/Naming. ppt download Brittle Ionic Compounds See the study guide on. summary of ionic compound properties. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. High melting and boiling points;. They conduct electricity when they are dissolved in water. It takes a large amount of mechanical force, such as striking a crystal with a. ionic compounds are hard. Brittle Ionic Compounds.
From www.youtube.com
Ionic Bonding Properties of Ionic Compounds YouTube Brittle Ionic Compounds It takes a large amount of mechanical force, such as striking a crystal with a. here are the properties of ionic compounds: Ionic compounds share several similar properties: ionic compounds are generally hard, but brittle. Ionic compounds generally have high. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature.. Brittle Ionic Compounds.
From slideplayer.com
Ionic and Covalent Compounds ppt download Brittle Ionic Compounds your starting assumption that ionic compounds are frequently brittle is misleading. summary of ionic compound properties. here are the properties of ionic compounds: High melting and boiling points;. the electrostatic repulsion can be enough to split the crystal, which is why ionic solids also are brittle. Many, if not most, solids are. It takes a large. Brittle Ionic Compounds.
From www.breakingatom.com
Ionic Properties Brittle Ionic Compounds High melting and boiling points;. See the study guide on. summary of ionic compound properties. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. They conduct electricity when they are dissolved in water. here are the properties of ionic compounds: Ionic compounds generally have high. Ionic compounds dissociate into. Brittle Ionic Compounds.