How To Prepare 10 Nitric Acid . to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. Visit byju's to understand the properties,. how to prepare 10 percent nitric acid solution? common acid solutions can be prepared using the handy table below. The third column lists the. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. in this video i will show you how to make a dilute solution of nitricacid with proper. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total.
from iqclasses.in
the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. common acid solutions can be prepared using the handy table below. The third column lists the. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Visit byju's to understand the properties,. in this video i will show you how to make a dilute solution of nitricacid with proper. how to prepare 10 percent nitric acid solution? for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total.
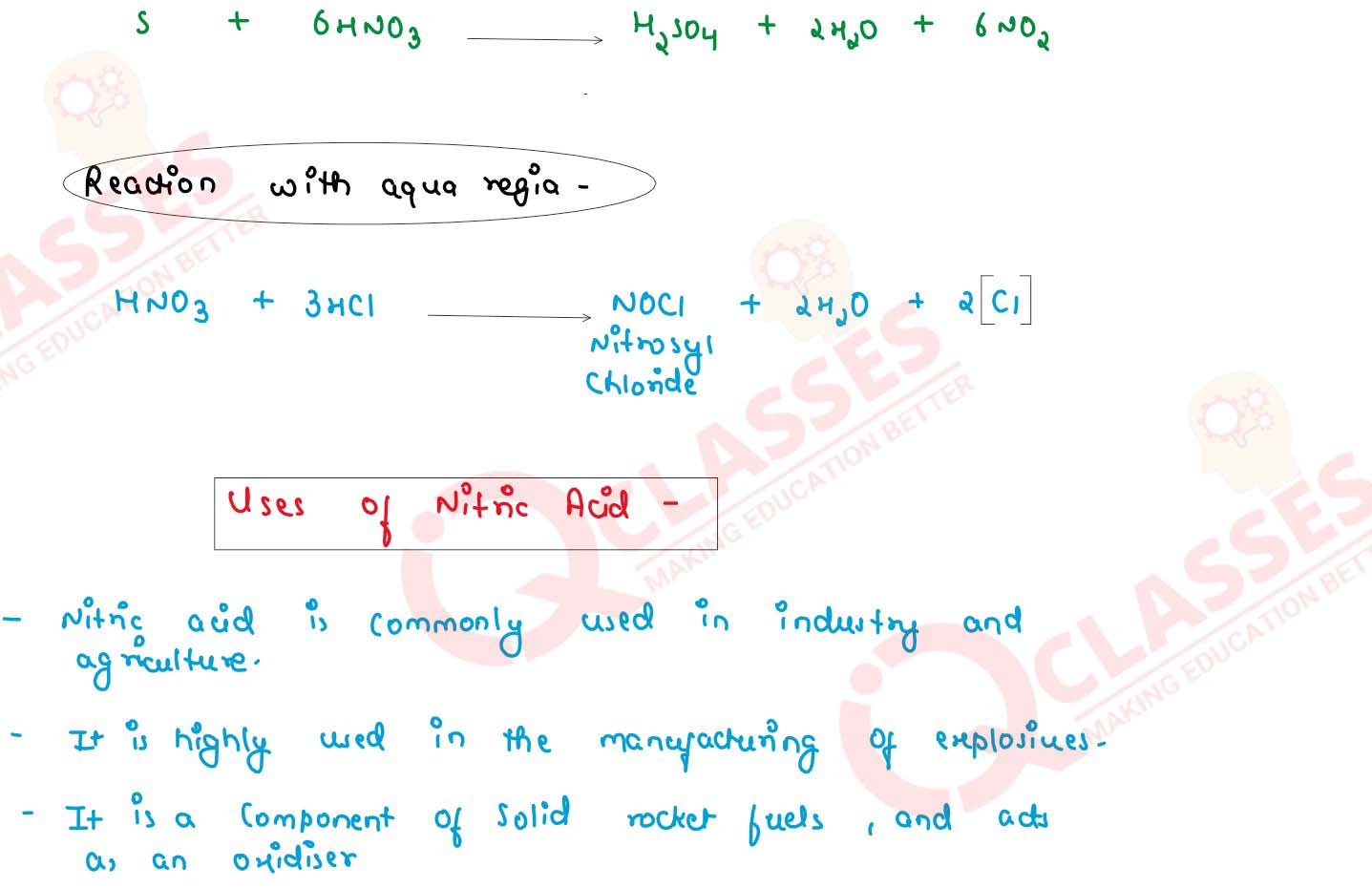
Class 10 ICSE Chemistry Important Notes Chapter Nitric Acid
How To Prepare 10 Nitric Acid Visit byju's to understand the properties,. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. how to prepare 10 percent nitric acid solution? The third column lists the. in this video i will show you how to make a dilute solution of nitricacid with proper. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. common acid solutions can be prepared using the handy table below. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. Visit byju's to understand the properties,. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or.
From www.numerade.com
SOLVED04 If the procedure outlined for this experiment has an overall How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually. How To Prepare 10 Nitric Acid.
From www.youtube.com
Reaction of Nitric Acid with Metals, Chemistry Lecture Sabaq.pk YouTube How To Prepare 10 Nitric Acid Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. Visit byju's to understand the properties,. in this video i will show you how to make a dilute solution of. How To Prepare 10 Nitric Acid.
From fasrpr700.weebly.com
How To Prepare 10 Nitric Acid fasrpr How To Prepare 10 Nitric Acid The third column lists the. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. how to prepare 10 percent nitric acid solution? the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. for example, a 70 %. How To Prepare 10 Nitric Acid.
From aslinter.weebly.com
How to prepare 10 nitric acid aslinter How To Prepare 10 Nitric Acid the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. Visit byju's to understand the properties,. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. how to prepare 10 percent nitric acid solution? to prepare 1000 ml. How To Prepare 10 Nitric Acid.
From www.myxxgirl.com
Nitric Acid For Analysis Emsure Milliporesigma Fisher Scientific My How To Prepare 10 Nitric Acid to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. Visit byju's to understand the properties,. how to prepare 10 percent nitric acid solution? in this video i will show you how to make a dilute solution of nitricacid with proper. . How To Prepare 10 Nitric Acid.
From www.youtube.com
Nitric Acid & it's Preparation Method YouTube How To Prepare 10 Nitric Acid to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. The third column lists the. in this. How To Prepare 10 Nitric Acid.
From www.youtube.com
Calculate the concentration of nitric acid in moles per litre in a How To Prepare 10 Nitric Acid Visit byju's to understand the properties,. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. in this video i will show you how to make a dilute solution of nitricacid with proper. the simplest and most used preparation of nitric acid. How To Prepare 10 Nitric Acid.
From www.sciencecompany.com
Reagent Grade 10 (v/v) Nitric Acid 500ml for sale. Buy from The How To Prepare 10 Nitric Acid in this video i will show you how to make a dilute solution of nitricacid with proper. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. the simplest and most used preparation of nitric acid in the lab is by adding. How To Prepare 10 Nitric Acid.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Nitric Acid How To Prepare 10 Nitric Acid in this video i will show you how to make a dilute solution of nitricacid with proper. Visit byju's to understand the properties,. how to prepare 10 percent nitric acid solution? for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. The. How To Prepare 10 Nitric Acid.
From iqclasses.in
Class 10 ICSE Chemistry Important Notes Chapter Nitric Acid How To Prepare 10 Nitric Acid to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. how to prepare 10 percent nitric acid solution? Visit byju's to understand the properties,. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. the simplest and most used. How To Prepare 10 Nitric Acid.
From www.youtube.com
Nitric Acid Synthesis YouTube How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? The third column lists the. common acid solutions can be prepared using the handy table below. in this video i will show you how to make a dilute solution of nitricacid with proper. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Visit byju's to. How To Prepare 10 Nitric Acid.
From medium.com
Fuming Nitric Acid Everything You Need To Know by Acid manufacturer How To Prepare 10 Nitric Acid in this video i will show you how to make a dilute solution of nitricacid with proper. common acid solutions can be prepared using the handy table below. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. The third column lists. How To Prepare 10 Nitric Acid.
From solutionpharmacy.in
How is nitric acid prepared? Solution Parmacy How To Prepare 10 Nitric Acid Visit byju's to understand the properties,. how to prepare 10 percent nitric acid solution? to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. common acid solutions can be prepared using the handy table below. in this video i will show. How To Prepare 10 Nitric Acid.
From www.youtube.com
Make Nitric acid(pure HNO3) by distillation using RETORT.Heating How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a. How To Prepare 10 Nitric Acid.
From www.alamy.com
Laboratory preparation of nitric (V) acid fully labeled diagram Stock How To Prepare 10 Nitric Acid Visit byju's to understand the properties,. common acid solutions can be prepared using the handy table below. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. how to. How To Prepare 10 Nitric Acid.
From mondolasopa242.weebly.com
How to prepare 10 nitric acid mondolasopa How To Prepare 10 Nitric Acid the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. The third column lists the. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. how. How To Prepare 10 Nitric Acid.
From www.scribd.com
Important Question ICSE 2010 Class 10th Nitric Acid PDF How To Prepare 10 Nitric Acid to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. Visit byju's to understand the properties,. the. How To Prepare 10 Nitric Acid.
From www.numerade.com
SOLVED What mass of a concentrated solution of nitric acid (68.0 HNO3 How To Prepare 10 Nitric Acid The third column lists the. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. common acid solutions can be prepared using the handy table below. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70. How To Prepare 10 Nitric Acid.
From hoakhoa.com.vn
Nitric acid 65 1004561000 How To Prepare 10 Nitric Acid The third column lists the. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Visit byju's to understand the properties,. in this video i will show you how. How To Prepare 10 Nitric Acid.
From owlcation.com
3 Ways to Prepare Nitric Acid Owlcation How To Prepare 10 Nitric Acid Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. in this video i will show you how to make a dilute solution of nitricacid with proper. common acid solutions can be prepared using the handy table below. The third column lists the. Visit byju's to understand the properties,. the simplest and most used. How To Prepare 10 Nitric Acid.
From owlcation.com
3 Ways to Prepare Nitric Acid Owlcation How To Prepare 10 Nitric Acid to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. The third column lists the. how to prepare 10 percent nitric acid solution? the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to. How To Prepare 10 Nitric Acid.
From www.echemi.com
How do I prepare 2 moles of a nitric acid solution? ECHEMI How To Prepare 10 Nitric Acid Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Visit byju's to understand the properties,. The third column lists the. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. in this video i will show you how to. How To Prepare 10 Nitric Acid.
From www.youtube.com
Make Nitric Acid at Home! Cheap and Easy DIY Method YouTube How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Visit byju's to understand the properties,. The third column lists the.. How To Prepare 10 Nitric Acid.
From askfilo.com
Formation of Nitric acid Nitric acid is formed by the combination of nitr.. How To Prepare 10 Nitric Acid for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. in this video i will show you how to make a dilute solution of nitricacid with proper. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to. How To Prepare 10 Nitric Acid.
From fasrpromos245.weebly.com
How To Prepare 10 Nitric Acid fasrpromos How To Prepare 10 Nitric Acid The third column lists the. for example, a 70 % (v/v) solution of ethanol can be prepared by dissolving 70 ml of 100% (i.e., 200 proof) ethanol in a total. common acid solutions can be prepared using the handy table below. Visit byju's to understand the properties,. the simplest and most used preparation of nitric acid in. How To Prepare 10 Nitric Acid.
From www.slideserve.com
PPT LECTURE (10) NITRIC ACID PRODUCTION 1INTRODUCTION PowerPoint How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? in this video i will show you how to make a dilute solution of nitricacid with proper. common acid solutions can be prepared using the handy table below. Visit byju's to understand the properties,. the simplest and most used preparation of nitric acid in the lab is by. How To Prepare 10 Nitric Acid.
From testbook.com
Uses of Nitric Acid Introduction, Daily Life Use, Industrial Use How To Prepare 10 Nitric Acid Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. Visit byju's to understand the properties,. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. The third column lists the. in this video i will show you how to. How To Prepare 10 Nitric Acid.
From www.youtube.com
Preparation of Nitric Acid in Laboratory Reaction HNO3 in the Lab How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. in this video i will show you how to make. How To Prepare 10 Nitric Acid.
From www.doubtnut.com
How many grams of concentrated nitric acid solution should be used to How To Prepare 10 Nitric Acid to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. common acid solutions can be prepared using the handy table below. for example, a 70 % (v/v) solution of. How To Prepare 10 Nitric Acid.
From aslinter.weebly.com
How to prepare 10 nitric acid aslinter How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. The third column lists the. common acid solutions can be prepared using the handy table below. for example, a 70 % (v/v) solution. How To Prepare 10 Nitric Acid.
From www.numerade.com
SOLVEDA nitric acid solution is found to have a pH of 2.70. Determine How To Prepare 10 Nitric Acid Visit byju's to understand the properties,. common acid solutions can be prepared using the handy table below. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. in. How To Prepare 10 Nitric Acid.
From www.youtube.com
Preparation of 1N Nitric Acid YouTube How To Prepare 10 Nitric Acid common acid solutions can be prepared using the handy table below. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. The third column lists the. Visit byju's to understand the properties,. in this video i will show you how to. How To Prepare 10 Nitric Acid.
From www.youtube.com
10ALab preparation of Nitric Acid YouTube How To Prepare 10 Nitric Acid Visit byju's to understand the properties,. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. Nitric acid (hno 3) is a clear, colorless to slightly yellow inorganic acid. how to prepare 10 percent nitric acid solution? for example, a 70. How To Prepare 10 Nitric Acid.
From lasopahh875.weebly.com
How to prepare 10 nitric acid lasopahh How To Prepare 10 Nitric Acid how to prepare 10 percent nitric acid solution? in this video i will show you how to make a dilute solution of nitricacid with proper. the simplest and most used preparation of nitric acid in the lab is by adding concentrated sulfuric acid to a dry nitrate salt (usually potassium or. Nitric acid (hno 3) is a. How To Prepare 10 Nitric Acid.
From www.youtube.com
Laboratory Preparation of Nitric Acid YouTube How To Prepare 10 Nitric Acid The third column lists the. to prepare 1000 ml of a 0.1 mol/l solution of nitric acid we have to dissolve 24.1433 g alcl3×6h2o (96 % purity) in deionized or. Visit byju's to understand the properties,. common acid solutions can be prepared using the handy table below. for example, a 70 % (v/v) solution of ethanol can. How To Prepare 10 Nitric Acid.