Zinc With Hydrochloric Acid Word Equation . zinc metal reacts with hydrochloric acid according to the balanced equation: this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. The hydrogen causes bubbling during the reaction, and can be detected using a burning. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. 2hcl + zn → zncl 2 + h 2. zinc reaction with hydrochloric acid. zinc reacts with halogens in the presence of moisture: an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. Zn + cl₂ → zncl₂. hydrochloric acid + zinc → zinc chloride + hydrogen.
from www.doubtnut.com
Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Zn + cl₂ → zncl₂. The hydrogen causes bubbling during the reaction, and can be detected using a burning. zinc metal reacts with hydrochloric acid according to the balanced equation: This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. hydrochloric acid + zinc → zinc chloride + hydrogen. zinc reacts with halogens in the presence of moisture: 2hcl + zn → zncl 2 + h 2. When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc.
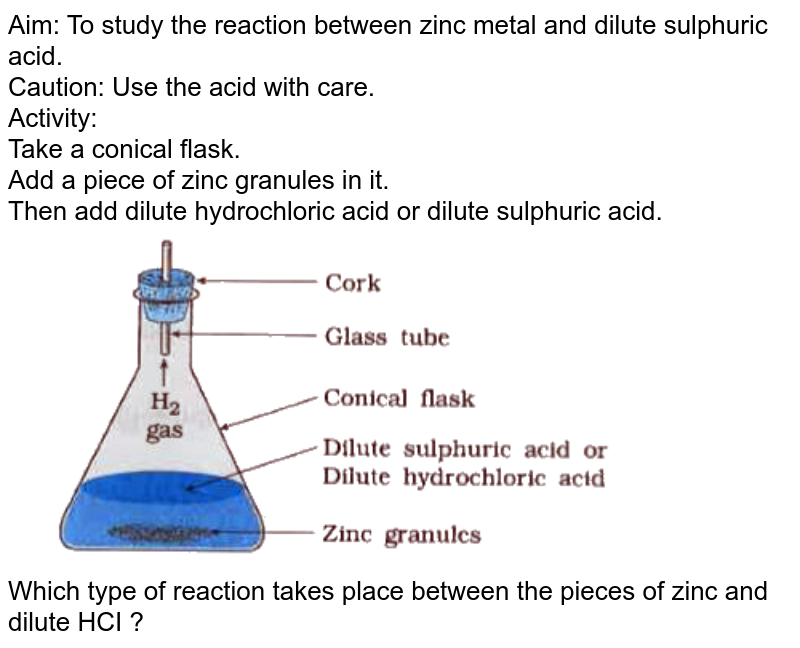
Write balanced chemical equation for the following 1. Action of
Zinc With Hydrochloric Acid Word Equation 2hcl + zn → zncl 2 + h 2. zinc reaction with hydrochloric acid. an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. hydrochloric acid + zinc → zinc chloride + hydrogen. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. The hydrogen causes bubbling during the reaction, and can be detected using a burning. 2hcl + zn → zncl 2 + h 2. Zn + cl₂ → zncl₂. zinc metal reacts with hydrochloric acid according to the balanced equation: When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. zinc reacts with halogens in the presence of moisture: this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc.
From kelasmenggambarbagus920.blogspot.com
Magnesium Oxide + Hydrochloric Acid Balanced Equation Now Zinc With Hydrochloric Acid Word Equation zinc metal reacts with hydrochloric acid according to the balanced equation: an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. Zn + cl₂ → zncl₂. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. zinc reaction with hydrochloric acid.. Zinc With Hydrochloric Acid Word Equation.
From chad-has-holt.blogspot.com
Zinc Reacts With Hydrochloric Acid ChadhasHolt Zinc With Hydrochloric Acid Word Equation zinc reaction with hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during. Zinc With Hydrochloric Acid Word Equation.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc With Hydrochloric Acid Word Equation an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. 2hcl + zn → zncl 2 + h 2. hydrochloric acid + zinc → zinc chloride + hydrogen. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with. Zinc With Hydrochloric Acid Word Equation.
From fity.club
Zinc Reacts With Hydrochloric Acid Zinc With Hydrochloric Acid Word Equation Zn + cl₂ → zncl₂. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. 2hcl + zn → zncl 2 + h 2. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. zinc reaction with hydrochloric acid. hydrochloric. Zinc With Hydrochloric Acid Word Equation.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Zinc With Hydrochloric Acid Word Equation When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. 2hcl + zn → zncl 2 + h 2. hydrochloric acid + zinc → zinc chloride + hydrogen. This reaction is commonly conducted in classrooms to. Zinc With Hydrochloric Acid Word Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc With Hydrochloric Acid Word Equation Zn + cl₂ → zncl₂. zinc reacts with halogens in the presence of moisture: When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. The hydrogen causes bubbling during the reaction, and can be detected using a burning. zinc reaction with. Zinc With Hydrochloric Acid Word Equation.
From brainly.com
Below is the word equation for the reaction between sodium hydroxide Zinc With Hydrochloric Acid Word Equation this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. Zn + cl₂ → zncl₂. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. The hydrogen causes bubbling during the reaction, and can be detected using a burning. 2hcl. Zinc With Hydrochloric Acid Word Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc With Hydrochloric Acid Word Equation hydrochloric acid + zinc → zinc chloride + hydrogen. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g). Zinc With Hydrochloric Acid Word Equation.
From www.doubtnut.com
Write balanced chemical equation for the following 1. Action of Zinc With Hydrochloric Acid Word Equation this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. zinc reacts with halogens in the presence of moisture: This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. 2hcl + zn → zncl. Zinc With Hydrochloric Acid Word Equation.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Zinc With Hydrochloric Acid Word Equation this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. 2hcl + zn → zncl 2 + h 2. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. zinc reaction with hydrochloric acid. zinc metal reacts with hydrochloric acid according to the balanced. Zinc With Hydrochloric Acid Word Equation.
From www.toppr.com
Zinc and hydrochloric acid react according to the reaction Zn(s Zinc With Hydrochloric Acid Word Equation This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. an explanation of. Zinc With Hydrochloric Acid Word Equation.
From www.alamy.com
Reaction of zinc with hydrochloric acid Stock Photo Alamy Zinc With Hydrochloric Acid Word Equation This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. Zn + cl₂ → zncl₂. hydrochloric acid + zinc → zinc chloride + hydrogen. When zinc reacts with hydrochloric acid, a. Zinc With Hydrochloric Acid Word Equation.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc With Hydrochloric Acid Word Equation 2hcl + zn → zncl 2 + h 2. Zn + cl₂ → zncl₂. an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. The hydrogen causes bubbling during the reaction, and can be detected using a burning. hydrochloric acid + zinc → zinc chloride + hydrogen. . Zinc With Hydrochloric Acid Word Equation.
From www.youtube.com
Zinc and 6 M Hydrochloric Acid Reaction YouTube Zinc With Hydrochloric Acid Word Equation hydrochloric acid + zinc → zinc chloride + hydrogen. an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs.. Zinc With Hydrochloric Acid Word Equation.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc With Hydrochloric Acid Word Equation an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. hydrochloric acid + zinc → zinc chloride + hydrogen. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. this chemistry video tutorial explains how to predict the products of the. Zinc With Hydrochloric Acid Word Equation.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Zinc With Hydrochloric Acid Word Equation Zn + cl₂ → zncl₂. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc reacts with halogens in the presence of moisture: 2hcl + zn → zncl 2 + h 2. an explanation of how to balance and write the the equation for zinc + hydrochloric and the. Zinc With Hydrochloric Acid Word Equation.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc With Hydrochloric Acid Word Equation hydrochloric acid + zinc → zinc chloride + hydrogen. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. an explanation of how to balance and write the the equation. Zinc With Hydrochloric Acid Word Equation.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc With Hydrochloric Acid Word Equation This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. this chemistry video tutorial explains how to predict the. Zinc With Hydrochloric Acid Word Equation.
From www.numerade.com
SOLVED A 5 gram sample of zinc is added to hydrochloric acid. The Zinc With Hydrochloric Acid Word Equation an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. The hydrogen causes bubbling during the reaction, and can be detected using a burning. this chemistry video tutorial explains how to predict. Zinc With Hydrochloric Acid Word Equation.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc With Hydrochloric Acid Word Equation zinc reaction with hydrochloric acid. When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the reaction, and can be detected using a burning. Zn + cl₂ → zncl₂. zinc reacts with halogens in the presence of moisture: hydrochloric acid + zinc. Zinc With Hydrochloric Acid Word Equation.
From www.numerade.com
SOLVED Write the equations for the following reactionsa.) Aluminium Zinc With Hydrochloric Acid Word Equation The hydrogen causes bubbling during the reaction, and can be detected using a burning. hydrochloric acid + zinc → zinc chloride + hydrogen. an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. Zn + cl₂ → zncl₂. This reaction is commonly conducted in classrooms to illustrate the. Zinc With Hydrochloric Acid Word Equation.
From readingandwritingprojectcom.web.fc2.com
zinc metal and hydrochloric acid Zinc With Hydrochloric Acid Word Equation 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the reaction, and can be detected using a burning. zinc reacts with halogens in the presence of moisture: this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. This reaction is commonly conducted in classrooms to illustrate. Zinc With Hydrochloric Acid Word Equation.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the reaction Zinc With Hydrochloric Acid Word Equation hydrochloric acid + zinc → zinc chloride + hydrogen. zinc reacts with halogens in the presence of moisture: this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc reaction with hydrochloric acid. Zn + cl₂ → zncl₂. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g). Zinc With Hydrochloric Acid Word Equation.
From www.nagwa.com
Question Video Identifying the Salt Produced in an AcidBase Reaction Zinc With Hydrochloric Acid Word Equation this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. zinc metal reacts. Zinc With Hydrochloric Acid Word Equation.
From ceojqkiy.blob.core.windows.net
Zinc Hydroxide Ionic Equation at Sharon Lyons blog Zinc With Hydrochloric Acid Word Equation 2hcl + zn → zncl 2 + h 2. an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. zinc reaction with hydrochloric acid. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103. Zinc With Hydrochloric Acid Word Equation.
From lessonlibremediably.z13.web.core.windows.net
Acid Base Reaction Explained Zinc With Hydrochloric Acid Word Equation Zn + cl₂ → zncl₂. The hydrogen causes bubbling during the reaction, and can be detected using a burning. 2hcl + zn → zncl 2 + h 2. an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. This reaction is commonly conducted in classrooms to illustrate the principles. Zinc With Hydrochloric Acid Word Equation.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Zinc With Hydrochloric Acid Word Equation This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. 2hcl + zn → zncl 2 + h 2. zinc reacts with halogens in the presence of moisture: Zn (s) + 2 hcl (aq)¡zncl2 (aq). Zinc With Hydrochloric Acid Word Equation.
From brainly.in
Write the chemical equation when zinc granules react with (a) Sulphuric Zinc With Hydrochloric Acid Word Equation Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. zinc metal reacts with hydrochloric acid according to the balanced equation: This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing. Zinc With Hydrochloric Acid Word Equation.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Zinc With Hydrochloric Acid Word Equation The hydrogen causes bubbling during the reaction, and can be detected using a burning. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. 2hcl + zn → zncl 2 + h 2. zinc reaction with hydrochloric acid. zinc reacts with halogens in the presence of moisture: Zn (s) +. Zinc With Hydrochloric Acid Word Equation.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Zinc With Hydrochloric Acid Word Equation Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc reacts with halogens in the presence of moisture: zinc metal reacts with hydrochloric acid according to the. Zinc With Hydrochloric Acid Word Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HCl = ZnCl2 + H2 YouTube Zinc With Hydrochloric Acid Word Equation Zn + cl₂ → zncl₂. This reaction is commonly conducted in classrooms to illustrate the principles of stoichiometry and balancing equations. zinc metal reacts with hydrochloric acid according to the balanced equation: The hydrogen causes bubbling during the reaction, and can be detected using a burning. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103. Zinc With Hydrochloric Acid Word Equation.
From www.youtube.com
Reaction of Zinc with dilute hydrochloric acid or sulphuric acid....Zn Zinc With Hydrochloric Acid Word Equation zinc reacts with halogens in the presence of moisture: zinc metal reacts with hydrochloric acid according to the balanced equation: an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s). Zinc With Hydrochloric Acid Word Equation.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc With Hydrochloric Acid Word Equation Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. zinc metal reacts with hydrochloric acid according to the balanced equation: an explanation of how to balance and write the the equation for zinc + hydrochloric and the correct coefficients for. this chemistry video tutorial. Zinc With Hydrochloric Acid Word Equation.
From www.nagwa.com
Question Video Understanding Oxidation and Reduction in the Reaction Zinc With Hydrochloric Acid Word Equation this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. hydrochloric acid + zinc → zinc chloride + hydrogen. The hydrogen causes bubbling during the reaction, and can be detected using a burning. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is. Zinc With Hydrochloric Acid Word Equation.
From www.youtube.com
How to Write the Net Ionic Equation for H2SO4 + ZnCO3 = ZnSO4 + CO2 Zinc With Hydrochloric Acid Word Equation When zinc reacts with hydrochloric acid, a fascinating chemical reaction occurs. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 ( g) when 0.103 g of zn (s) is combined with enough hcl. zinc reaction with hydrochloric acid. this chemistry video tutorial explains how to predict the products of the single replacement reaction between zinc. zinc reacts. Zinc With Hydrochloric Acid Word Equation.