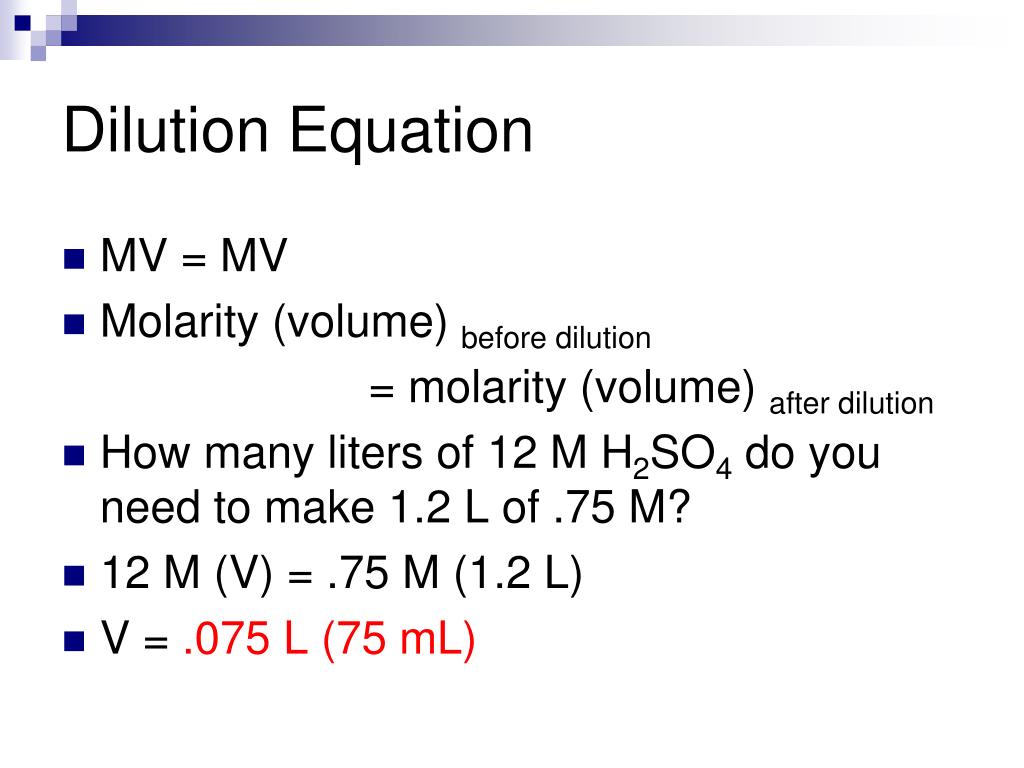
Dilution Formula Derivation . Often, a worker will need to change the concentration of a solution by changing the. We can also use dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the. 50 μl per well * 2 for. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Learn how to dilute and concentrate solutions. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Calculate the minimum diluent volume per step: What is the equation and formula for finding dilution? To make a dilution series, use the following formulas:
from www.slideserve.com
To make a dilution series, use the following formulas: What is the equation and formula for finding dilution? Concentration is the removal of solvent, which increases the. Learn how to dilute and concentrate solutions. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. State whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. 50 μl per well * 2 for. Calculate the minimum diluent volume per step:
PPT Solubility PowerPoint Presentation, free download ID2801194
Dilution Formula Derivation The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. What is the equation and formula for finding dilution? Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Learn how to dilute and concentrate solutions. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. We can also use dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. To make a dilution series, use the following formulas: 50 μl per well * 2 for. Calculate the minimum diluent volume per step:
From brainly.in
derive ostwald's dilution law for weak acid ch3cooh Brainly.in Dilution Formula Derivation To make a dilution series, use the following formulas: What is the equation and formula for finding dilution? Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. We can also use dilution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Calculate the minimum. Dilution Formula Derivation.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Formula Derivation Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. What is the. Dilution Formula Derivation.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Formula Derivation 50 μl per well * 2 for. State whether the concentration of a solution is directly or indirectly proportional to its volume. What is the equation and formula for finding dilution? Calculate the minimum diluent volume per step: Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which. Dilution Formula Derivation.
From ar.inspiredpencil.com
Dilution Equation Dilution Formula Derivation Concentration is the removal of solvent, which increases the. Learn how to dilute and concentrate solutions. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the. Calculate the minimum diluent volume per step: Dilution is the addition. Dilution Formula Derivation.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Formula Derivation 50 μl per well * 2 for. To make a dilution series, use the following formulas: We can also use dilution. What is the equation and formula for finding dilution? Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration. Dilution Formula Derivation.
From www.youtube.com
Dilution in biology lab How to use the equation C1V1 = C2V2 YouTube Dilution Formula Derivation Learn how to dilute and concentrate solutions. We can also use dilution. What is the equation and formula for finding dilution? Concentration is the removal of solvent, which increases the. Calculate the minimum diluent volume per step: Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is. Dilution Formula Derivation.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube Dilution Formula Derivation What is the equation and formula for finding dilution? The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Concentration is the removal of solvent, which increases the. Often, a worker will need to change the concentration of a solution by changing the. The equation or formula for finding dilutions are m1v1=m2v2. Dilution Formula Derivation.
From www.slideserve.com
PPT Making Molar Solutions PowerPoint Presentation, free download Dilution Formula Derivation What is the equation and formula for finding dilution? The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute. Dilution Formula Derivation.
From www.freepik.com
Premium Vector Dilution factor formula science vector illustration Dilution Formula Derivation To make a dilution series, use the following formulas: The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Often, a worker will need to change the concentration of a solution by changing the. Calculate the minimum diluent volume per step: State whether the concentration of a solution is directly or indirectly. Dilution Formula Derivation.
From www.toppr.com
Deduce equation for Ostwalds dilution law with respect to the weak acid. Dilution Formula Derivation The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of solvent, which increases the. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. The dilution. Dilution Formula Derivation.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Formula Derivation What is the equation and formula for finding dilution? The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. 50 μl per well * 2 for. The equation or formula for finding dilutions are m1v1=m2v2 or one. Dilution Formula Derivation.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Formula Derivation Calculate the minimum diluent volume per step: What is the equation and formula for finding dilution? The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. State whether the concentration of a solution is. Dilution Formula Derivation.
From chem2u.blogspot.com
chem2U Dilution method formula Dilution Formula Derivation The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. The dilution equation is a simple relation between concentrations and volumes. Dilution Formula Derivation.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Formula Derivation Concentration is the removal of solvent, which increases the. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The dilution equation is a simple relation between concentrations and volumes of a. Dilution Formula Derivation.
From www.slideserve.com
PPT Chapter 11 Measuring Solubility PowerPoint Presentation, free Dilution Formula Derivation What is the equation and formula for finding dilution? Calculate the minimum diluent volume per step: We can also use dilution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Concentration is the removal of solvent, which increases the. 50 μl per well * 2 for. Dilution is the addition of. Dilution Formula Derivation.
From www.slideserve.com
PPT Molarity & Dilution PowerPoint Presentation, free download ID Dilution Formula Derivation State whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. We can also use dilution. 50 μl per well * 2 for. Concentration is the removal of solvent, which increases the. What is the equation and formula. Dilution Formula Derivation.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Formula Derivation Often, a worker will need to change the concentration of a solution by changing the. To make a dilution series, use the following formulas: The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. What is the equation and formula for finding dilution? We can also use dilution. 50 μl per well. Dilution Formula Derivation.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Dilution Formula Derivation Calculate the minimum diluent volume per step: To make a dilution series, use the following formulas: What is the equation and formula for finding dilution? State whether the concentration of a solution is directly or indirectly proportional to its volume. 50 μl per well * 2 for. The dilution equation is a simple relation between concentrations and volumes of a. Dilution Formula Derivation.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Formula Derivation 50 μl per well * 2 for. We can also use dilution. To make a dilution series, use the following formulas: Often, a worker will need to change the concentration of a solution by changing the. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. Concentration is the removal of solvent, which increases. Dilution Formula Derivation.
From www.scribd.com
Dilution (Equation) Wikipedia PDF Solution Chemistry Dilution Formula Derivation Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which increases the. Learn how to dilute and concentrate solutions. We can also use dilution. To make a dilution series, use the following formulas: What is the equation and formula for finding dilution? The equation or formula for. Dilution Formula Derivation.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Formula Derivation Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To make a dilution series, use the following formulas: Often, a worker will need to change the concentration of a solution by changing the. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. 50 μl. Dilution Formula Derivation.
From www.youtube.com
Dilution Equation 1 YouTube Dilution Formula Derivation What is the equation and formula for finding dilution? Concentration is the removal of solvent, which increases the. 50 μl per well * 2 for. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. To. Dilution Formula Derivation.
From www.slideshare.net
Respiration2 Dilution Formula Derivation Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Calculate. Dilution Formula Derivation.
From www.chegg.com
Q6. a) Derive the equation of helium dilution method Dilution Formula Derivation The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Often, a worker will need to change the concentration of a solution by changing the. We can also use dilution. To make a dilution series, use the following formulas: Learn how to dilute and concentrate solutions. State whether the concentration of a. Dilution Formula Derivation.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Formula Derivation The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. To make a dilution series, use the following formulas: State whether the concentration of a solution is directly or indirectly. Dilution Formula Derivation.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Formula Derivation Often, a worker will need to change the concentration of a solution by changing the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Calculate the minimum diluent volume per step: State whether the concentration of a solution is directly or indirectly proportional to its volume. The equation or formula for finding dilutions. Dilution Formula Derivation.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Formula Derivation Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. To make a dilution series, use the following formulas: The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Concentration is the removal of solvent, which increases the. What is the equation and formula for finding. Dilution Formula Derivation.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Formula Derivation The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. 50 μl per well * 2 for. Concentration is the removal of solvent, which increases the. The equation or. Dilution Formula Derivation.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Dilution Formula Derivation The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. To make a dilution series, use the following formulas: We can also use dilution. What is the equation and formula for finding dilution? State whether the. Dilution Formula Derivation.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution Formula Derivation Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. What is the equation and formula for finding dilution? The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. The equation or formula for finding dilutions are m1v1=m2v2 or. Dilution Formula Derivation.
From paghis.weebly.com
Easy way to do dilutions paghis Dilution Formula Derivation The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. We can also use dilution. Calculate the minimum diluent volume per step: Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. 50 μl per well * 2 for. Dilution. Dilution Formula Derivation.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2801194 Dilution Formula Derivation What is the equation and formula for finding dilution? Calculate the minimum diluent volume per step: 50 μl per well * 2 for. Concentration is the removal of solvent, which increases the. Learn how to dilute and concentrate solutions. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution. We can also. Dilution Formula Derivation.
From www.slideshare.net
Molarity and dilution Dilution Formula Derivation Calculate the minimum diluent volume per step: State whether the concentration of a solution is directly or indirectly proportional to its volume. We can also use dilution. What is the equation and formula for finding dilution? The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. Concentration is the removal of solvent,. Dilution Formula Derivation.
From www.slideserve.com
PPT Chapter 1 The Organization of Matter PowerPoint Presentation Dilution Formula Derivation Often, a worker will need to change the concentration of a solution by changing the. Learn how to dilute and concentrate solutions. What is the equation and formula for finding dilution? State whether the concentration of a solution is directly or indirectly proportional to its volume. We can also use dilution. The solute concentration of a solution may be decreased. Dilution Formula Derivation.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Formula Derivation State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the. The equation or formula for finding dilutions are m1v1=m2v2 or one of the concentration formulas derived. Concentration is the removal of solvent, which. Dilution Formula Derivation.