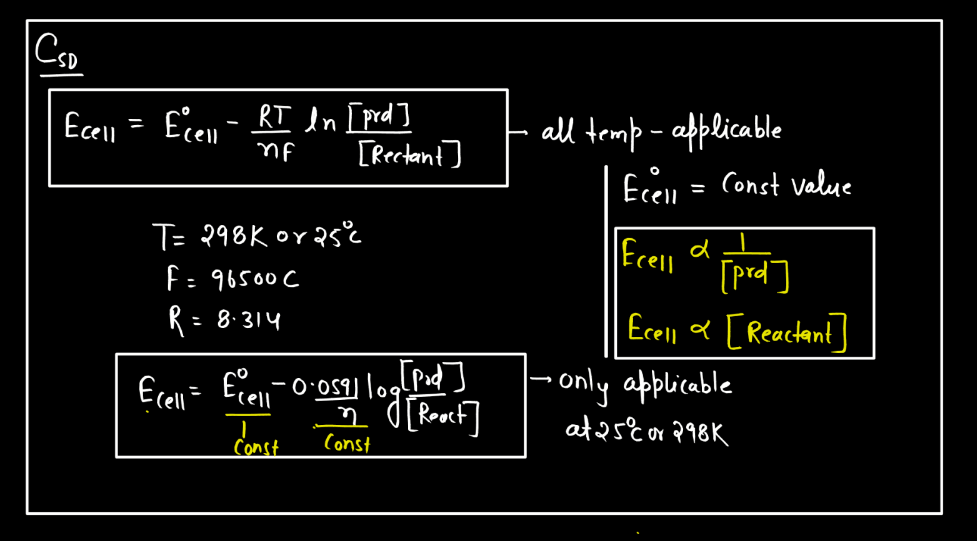
Electrode Potential Definition A Levels . Revise how to measure standard electrode potential and how to construct an overall redox equation. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Then, test yourself with our exam questions. This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. The convention is to put the more positive. Electrode potentials and the electrochemical series: This value is called the electrode potential (e) and is measured in volts. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Electrochemical cells use redox reactions as the electron transfer between products creates a. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell.
from www.careerpower.in
Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. This value is called the electrode potential (e) and is measured in volts. Electrode potentials and the electrochemical series: The convention is to put the more positive. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. Electrochemical cells use redox reactions as the electron transfer between products creates a. This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. Revise how to measure standard electrode potential and how to construct an overall redox equation.
Electrode Potential Definition, Formula, Standard Electrode Potential
Electrode Potential Definition A Levels Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Electrochemical cells use redox reactions as the electron transfer between products creates a. The convention is to put the more positive. This value is called the electrode potential (e) and is measured in volts. Then, test yourself with our exam questions. Electrode potentials and the electrochemical series: Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. An introduction to redox equilibria and electrode potentials. This page explains the background to standard electrode potentials (redox potentials), showing how they. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Revise how to measure standard electrode potential and how to construct an overall redox equation.
From www.studypool.com
SOLUTION Standard electrode potential definition significance example Electrode Potential Definition A Levels This page explains the background to standard electrode potentials (redox potentials), showing how they. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. The convention is to put the more positive.. Electrode Potential Definition A Levels.
From www.youtube.com
Electrode Potentials and Cells Everything You NEED To Know|AQA A Electrode Potential Definition A Levels Then, test yourself with our exam questions. The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. This page explains the background to standard electrode potentials (redox potentials), showing how they. This value is called the electrode potential (e) and is measured in volts. Standard electrode potentials,. Electrode Potential Definition A Levels.
From greenenergymaterial.com
What Is Electrode Potential And Their Applications » Green Energy Material Electrode Potential Definition A Levels Then, test yourself with our exam questions. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Electrode potentials and the electrochemical series: The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Revise how. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels Then, test yourself with our exam questions. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. This page explains the background to standard electrode potentials (redox potentials), showing how they. Revise how to measure standard electrode potential and how to construct an overall redox equation. An introduction. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels Revise how to measure standard electrode potential and how to construct an overall redox equation. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. This value is called the electrode potential. Electrode Potential Definition A Levels.
From www.tes.com
AQA ALevel Chemistry Electrode Potentials and Electrochemical Cells Electrode Potential Definition A Levels Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Revise how to measure standard electrode potential. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels This value is called the electrode potential (e) and is measured in volts. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Electrode potentials and the electrochemical series: Electrode potentials can. Electrode Potential Definition A Levels.
From youtube.com
19.1.4 Predict whether a reaction will be spontaneous using standard Electrode Potential Definition A Levels This value is called the electrode potential (e) and is measured in volts. Electrode potentials and the electrochemical series: Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Electrochemical cells use redox reactions as the electron transfer between products creates a. The convention is to put the more positive. When an electrode is dipped. Electrode Potential Definition A Levels.
From www.youtube.com
CHEMISTRY A LEVEL ELECTRODE POTENTIAL YouTube Electrode Potential Definition A Levels The convention is to put the more positive. Electrochemical cells use redox reactions as the electron transfer between products creates a. Revise how to measure standard electrode potential and how to construct an overall redox equation. This value is called the electrode potential (e) and is measured in volts. This page explains the background to standard electrode potentials (redox potentials),. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels This page explains the background to standard electrode potentials (redox potentials), showing how they. An introduction to redox equilibria and electrode potentials. The convention is to put the more positive. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. When an electrode is dipped into an electrolyte solution, a potential difference is established. Electrode Potential Definition A Levels.
From question.pandai.org
Standard Electrode Potential Electrode Potential Definition A Levels Electrode potentials and the electrochemical series: Electrochemical cells use redox reactions as the electron transfer between products creates a. An introduction to redox equilibria and electrode potentials. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. This value is called the electrode potential (e) and is measured in volts. Electrode potentials can be. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels The convention is to put the more positive. Electrode potentials and the electrochemical series: An introduction to redox equilibria and electrode potentials. This value is called the electrode potential (e) and is measured in volts. This page explains the background to standard electrode potentials (redox potentials), showing how they. Electrochemical cells use redox reactions as the electron transfer between products. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Electrochemical cells use redox reactions as the electron transfer between products creates a. Then, test yourself with our exam questions. Electrode potentials and the electrochemical series: Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode. Electrode Potential Definition A Levels.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Electrode Potential Definition A Levels Then, test yourself with our exam questions. Electrode potentials and the electrochemical series: This value is called the electrode potential (e) and is measured in volts. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. This page explains the background to standard electrode potentials (redox potentials), showing how they. The convention is to. Electrode Potential Definition A Levels.
From chemistnotes.com
Single electrode potential Definition, origin, expression Chemistry Electrode Potential Definition A Levels The convention is to put the more positive. Revise how to measure standard electrode potential and how to construct an overall redox equation. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. Then, test yourself with our exam questions. An introduction to redox equilibria and electrode potentials. Electrochemical cells use redox reactions as. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels An introduction to redox equilibria and electrode potentials. The convention is to put the more positive. This page explains the background to standard electrode potentials (redox potentials), showing how they. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. When an electrode is dipped into an electrolyte solution, a potential difference is established between. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels Electrochemical cells use redox reactions as the electron transfer between products creates a. Electrode potentials and the electrochemical series: Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. This value is called the electrode potential (e) and is measured in volts. When an electrode is dipped into an electrolyte solution, a potential difference. Electrode Potential Definition A Levels.
From www.tes.com
Revision mindmap for AQA A level electrode potentials Teaching Resources Electrode Potential Definition A Levels Electrochemical cells use redox reactions as the electron transfer between products creates a. This value is called the electrode potential (e) and is measured in volts. An introduction to redox equilibria and electrode potentials. The convention is to put the more positive. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels Revise how to measure standard electrode potential and how to construct an overall redox equation. An introduction to redox equilibria and electrode potentials. This value is called the electrode potential (e) and is measured in volts. The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Electrochemical. Electrode Potential Definition A Levels.
From in.pinterest.com
Electrode Potential Energy Definition, Formula and Example Potential Electrode Potential Definition A Levels Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. Electrode potentials and the electrochemical series: Then, test yourself with our exam questions. Electrochemical cells use redox reactions as the electron transfer between products creates a. This value is called the electrode potential (e) and is measured in volts. An introduction to redox equilibria. Electrode Potential Definition A Levels.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Electrode Potential Definition A Levels When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a. Electrode Potential Definition A Levels.
From www.youtube.com
APSC132 lecture 5 03 Standard Electrode Potential YouTube Electrode Potential Definition A Levels This value is called the electrode potential (e) and is measured in volts. The convention is to put the more positive. Electrochemical cells use redox reactions as the electron transfer between products creates a. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. This page explains the. Electrode Potential Definition A Levels.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Electrode Potential Definition A Levels This value is called the electrode potential (e) and is measured in volts. Electrochemical cells use redox reactions as the electron transfer between products creates a. Then, test yourself with our exam questions. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. An introduction to redox equilibria and electrode potentials. Electrode potentials can. Electrode Potential Definition A Levels.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Potential Definition A Levels The convention is to put the more positive. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. This value is called the electrode potential (e) and is measured in volts. Revise how to measure standard electrode potential and how to construct an overall redox equation. Standard electrode potentials, standard hydrogen electrode and how to. Electrode Potential Definition A Levels.
From www.youtube.com
Electrode Potentials Electrochemical Series A level Chemistry Electrode Potential Definition A Levels Electrode potentials and the electrochemical series: This page explains the background to standard electrode potentials (redox potentials), showing how they. This value is called the electrode potential (e) and is measured in volts. Revise how to measure standard electrode potential and how to construct an overall redox equation. An introduction to redox equilibria and electrode potentials. The convention is to. Electrode Potential Definition A Levels.
From www.slideshare.net
Electrode potential and its applications Electrode Potential Definition A Levels Revise how to measure standard electrode potential and how to construct an overall redox equation. Then, test yourself with our exam questions. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. This page explains the background to standard electrode potentials (redox potentials), showing how they. Electrochemical cells. Electrode Potential Definition A Levels.
From www.youtube.com
Electrochemistry Part 10 A Levels 9701 Nonstandard Electrode Electrode Potential Definition A Levels Electrochemical cells use redox reactions as the electron transfer between products creates a. This value is called the electrode potential (e) and is measured in volts. Revise how to measure standard electrode potential and how to construct an overall redox equation. The convention is to put the more positive. Then, test yourself with our exam questions. Electrode potentials can be. Electrode Potential Definition A Levels.
From www.pinterest.com
Electrode Potential Easy Science Flashcards, Electrodes Electrode Potential Definition A Levels Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Electrode potentials and the electrochemical series: Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. Electrochemical cells use redox reactions as the electron transfer between products creates a. The convention is to put the more positive. An introduction. Electrode Potential Definition A Levels.
From www.researchgate.net
4 Energy and potential levels of the electrode/electrolyte interface Electrode Potential Definition A Levels The convention is to put the more positive. Then, test yourself with our exam questions. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Revise how to measure standard electrode potential and how to construct an overall redox equation. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a. Electrode Potential Definition A Levels.
From scienceinfo.com
Electrode Potential Types, Importance, and Applications Electrode Potential Definition A Levels Revise how to measure standard electrode potential and how to construct an overall redox equation. Electrochemical cells use redox reactions as the electron transfer between products creates a. This page explains the background to standard electrode potentials (redox potentials), showing how they. Then, test yourself with our exam questions. Electrode potentials and the electrochemical series: The convention is to put. Electrode Potential Definition A Levels.
From testbook.com
Standard Electrode PotentialLearn Definition,Formula,Conditions Electrode Potential Definition A Levels Then, test yourself with our exam questions. Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. The convention is to put the more positive. Revise how to measure standard electrode potential and how to construct an overall redox equation. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a. Electrode Potential Definition A Levels.
From 2012books.lardbucket.org
Standard Potentials Electrode Potential Definition A Levels Electrode potentials can be used to calculate the voltage produced when electrodes are paired up. Then, test yourself with our exam questions. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. This value is called the electrode potential (e) and is measured in volts. An introduction to redox equilibria and electrode potentials. Electrode. Electrode Potential Definition A Levels.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Electrode Potential Definition A Levels Then, test yourself with our exam questions. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. Revise how to measure standard electrode potential and how to construct an overall redox equation. An introduction to redox equilibria and electrode potentials. Electrode potentials and the electrochemical series: Electrode potentials can be used to calculate the. Electrode Potential Definition A Levels.
From www.geeksforgeeks.org
Standard Electrode Potential Definition, Formula, Construction, Uses Electrode Potential Definition A Levels Electrochemical cells use redox reactions as the electron transfer between products creates a. When an electrode is dipped into an electrolyte solution, a potential difference is established between the electrode and solution termed electrode potential. Revise how to measure standard electrode potential and how to construct an overall redox equation. Electrode potentials can be used to calculate the voltage produced. Electrode Potential Definition A Levels.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Electrode Potential Definition A Levels Revise how to measure standard electrode potential and how to construct an overall redox equation. Electrode potentials and the electrochemical series: This value is called the electrode potential (e) and is measured in volts. Standard electrode potentials, standard hydrogen electrode and how to calculate the electrode potential for a cell. An introduction to redox equilibria and electrode potentials. Then, test. Electrode Potential Definition A Levels.