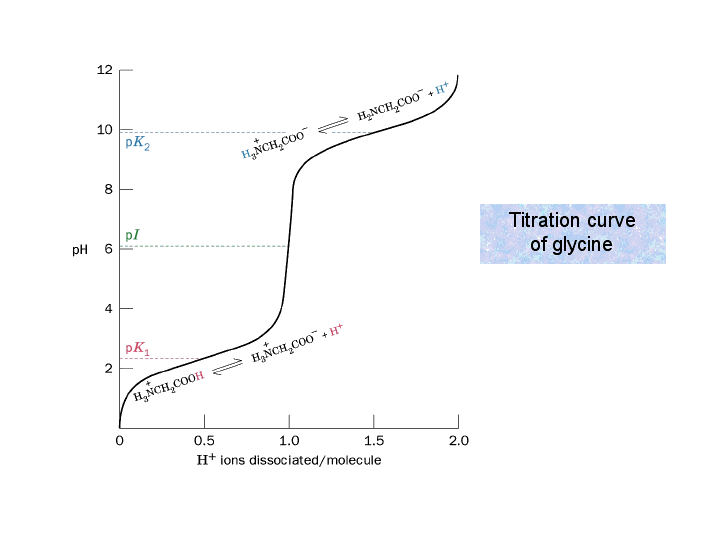
What Titration Curve Of Glycine . The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. Undergraduate biochemistry students should have great familiarity with titration curves. Titration curves of amino acids are very useful for identification. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. The isoelectric points range from 5.5 to 6.2. In order to understand this. As you can see in the example for glycine shown below, a simple. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. Below is a typical curve for the titration of glycine with naoh. These curves allow the prediction of protonation states, charges, and isoelectric points. The titration curve for glycine looks like the titration curve for a weak diprotic acid.
from www3.nd.edu
Titration curves of amino acids are very useful for identification. In order to understand this. The titration curve for glycine looks like the titration curve for a weak diprotic acid. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. The isoelectric points range from 5.5 to 6.2. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. As you can see in the example for glycine shown below, a simple. Undergraduate biochemistry students should have great familiarity with titration curves. Below is a typical curve for the titration of glycine with naoh. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point.
Titration curve of glycine
What Titration Curve Of Glycine Titration curves of amino acids are very useful for identification. In order to understand this. These curves allow the prediction of protonation states, charges, and isoelectric points. Undergraduate biochemistry students should have great familiarity with titration curves. Titration curves of amino acids are very useful for identification. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. The titration curve for glycine looks like the titration curve for a weak diprotic acid. Below is a typical curve for the titration of glycine with naoh. As you can see in the example for glycine shown below, a simple. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. The isoelectric points range from 5.5 to 6.2.
From www.researchgate.net
Titration curves (M= Nickel, L 1 = Glycine, L 2 = PAB, A= HNO 3 What Titration Curve Of Glycine The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. Undergraduate biochemistry students should have great familiarity with titration curves. Below is a typical curve for the titration of glycine with naoh. The figure below shows what happens to the ph of an. What Titration Curve Of Glycine.
From ar.inspiredpencil.com
Glycine Titration Curve What Titration Curve Of Glycine Titration curves of amino acids are very useful for identification. These curves allow the prediction of protonation states, charges, and isoelectric points. The isoelectric points range from 5.5 to 6.2. Below is a typical curve for the titration of glycine with naoh. Undergraduate biochemistry students should have great familiarity with titration curves. The results of these equations and the creation. What Titration Curve Of Glycine.
From www.youtube.com
Titration Curve for glycine in a easiest way YouTube What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. The isoelectric points range from 5.5 to 6.2. These curves allow the prediction of protonation states, charges, and isoelectric points. Undergraduate biochemistry students should have great familiarity with titration curves. Titration curves of amino acids are very useful for identification. In order to understand this. As you can. What Titration Curve Of Glycine.
From ar.inspiredpencil.com
Glycine Titration Curve What Titration Curve Of Glycine As you can see in the example for glycine shown below, a simple. Titration curves of amino acids are very useful for identification. The isoelectric points range from 5.5 to 6.2. The titration curve for glycine looks like the titration curve for a weak diprotic acid. In order to understand this. The figure below shows what happens to the ph. What Titration Curve Of Glycine.
From www.youtube.com
Titration curve of Glycine YouTube What Titration Curve Of Glycine The isoelectric points range from 5.5 to 6.2. The titration curve for glycine looks like the titration curve for a weak diprotic acid. In order to understand this. Titration curves of amino acids are very useful for identification. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with. What Titration Curve Of Glycine.
From www.transtutors.com
(Solved) Shown below is the titration curve of glycine (Gly), using What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. The isoelectric points range from 5.5 to 6.2. Undergraduate biochemistry students should have great familiarity with titration curves. The results of these equations and the creation of a titration. What Titration Curve Of Glycine.
From chempedia.info
Glycine titration curve Big Chemical Encyclopedia What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. In order to understand this. As you can see in the example for glycine shown below, a simple. The titration. What Titration Curve Of Glycine.
From www.chegg.com
1)According to the titration curve of glycine shown What Titration Curve Of Glycine The isoelectric points range from 5.5 to 6.2. The titration curve for glycine looks like the titration curve for a weak diprotic acid. As you can see in the example for glycine shown below, a simple. Below is a typical curve for the titration of glycine with naoh. In order to understand this. Titration curves of amino acids are very. What Titration Curve Of Glycine.
From www.pinterest.com
Glycine Titration Curve And Its Steps Biochemistry, Curve, Medical What Titration Curve Of Glycine The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. The titration curve for glycine looks like the titration curve for a weak diprotic acid. The isoelectric points range from 5.5 to 6.2. The titration curve of glycine provides vital information about. What Titration Curve Of Glycine.
From www.chegg.com
Solved Given below is the titration curve of glycine. The What Titration Curve Of Glycine Undergraduate biochemistry students should have great familiarity with titration curves. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. In order to understand this. The isoelectric points range from 5.5 to 6.2. Titration curves of amino acids are very useful for identification. The results of these equations and the creation. What Titration Curve Of Glycine.
From www.vedantu.com
What is the titration curve of glycine? What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. The isoelectric points range from 5.5 to 6.2. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated. What Titration Curve Of Glycine.
From www.studypool.com
SOLUTION Glycine titration curve complete concept Studypool What Titration Curve Of Glycine As you can see in the example for glycine shown below, a simple. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. Titration curves of amino acids are very useful for identification. Undergraduate biochemistry students should have great familiarity with titration. What Titration Curve Of Glycine.
From www.studypool.com
SOLUTION Glycine titration curve complete concept Studypool What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. These curves allow the prediction of protonation states, charges, and isoelectric points. Undergraduate biochemistry students should have great familiarity with titration curves. The isoelectric points range from 5.5 to 6.2. The results of these equations and the creation of a titration curve will signify that glycine is a. What Titration Curve Of Glycine.
From www.chegg.com
Solved The curve below represents the titration of glycine What Titration Curve Of Glycine These curves allow the prediction of protonation states, charges, and isoelectric points. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. In order to understand this. The isoelectric points range from 5.5 to 6.2. The titration curve for glycine looks like. What Titration Curve Of Glycine.
From www.youtube.com
Titration Curve of Glycine YouTube What Titration Curve Of Glycine These curves allow the prediction of protonation states, charges, and isoelectric points. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. As you can see in the example for glycine shown below, a simple. The results of these equations and the creation of a titration curve will signify that glycine. What Titration Curve Of Glycine.
From www.chegg.com
Solved A titration curve of glycine is shown below Where What Titration Curve Of Glycine The isoelectric points range from 5.5 to 6.2. These curves allow the prediction of protonation states, charges, and isoelectric points. Below is a typical curve for the titration of glycine with naoh. In order to understand this. Undergraduate biochemistry students should have great familiarity with titration curves. The results of these equations and the creation of a titration curve will. What Titration Curve Of Glycine.
From biochemden.com
Titration Curve of Glycine The zwitter ionic changes What Titration Curve Of Glycine The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. As you can see in. What Titration Curve Of Glycine.
From www.chegg.com
Solved Examine the titration curve for glycine, starting What Titration Curve Of Glycine These curves allow the prediction of protonation states, charges, and isoelectric points. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. Below is a typical curve for the titration of glycine with naoh. As you can see in the example for glycine. What Titration Curve Of Glycine.
From www.chegg.com
Solved Examine the titration curve for glycine, starting What Titration Curve Of Glycine In order to understand this. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral.. What Titration Curve Of Glycine.
From www.numerade.com
SOLVED 5. Shown below is the titration curve of glycine (Gly), using What Titration Curve Of Glycine Undergraduate biochemistry students should have great familiarity with titration curves. These curves allow the prediction of protonation states, charges, and isoelectric points. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. The titration curve of glycine provides vital information about its basic. What Titration Curve Of Glycine.
From ar.inspiredpencil.com
Glycine Titration Curve What Titration Curve Of Glycine Undergraduate biochemistry students should have great familiarity with titration curves. Titration curves of amino acids are very useful for identification. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. In order to understand this. The titration curve for glycine looks like the titration curve for a weak diprotic acid. The. What Titration Curve Of Glycine.
From www.slideserve.com
PPT Amino acids PowerPoint Presentation ID6032806 What Titration Curve Of Glycine In order to understand this. These curves allow the prediction of protonation states, charges, and isoelectric points. Undergraduate biochemistry students should have great familiarity with titration curves. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. The figure below shows what happens to the ph of an acidic solution of. What Titration Curve Of Glycine.
From www.numerade.com
SOLVED The diagram below represents a titration curve of glycine What Titration Curve Of Glycine The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. Titration curves of amino acids are very useful for identification. The titration curve for glycine looks like the titration curve for a weak diprotic acid. The titration curve of glycine provides vital information. What Titration Curve Of Glycine.
From chart-studio.plotly.com
Titration Curve of Glycine HCl scatter chart made by Cvaltier plotly What Titration Curve Of Glycine These curves allow the prediction of protonation states, charges, and isoelectric points. As you can see in the example for glycine shown below, a simple. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. Undergraduate biochemistry students should have great familiarity with. What Titration Curve Of Glycine.
From www.researchgate.net
Potentiometric titration curves for the Zn(II)lcysteineglycine What Titration Curve Of Glycine The isoelectric points range from 5.5 to 6.2. These curves allow the prediction of protonation states, charges, and isoelectric points. The titration curve for glycine looks like the titration curve for a weak diprotic acid. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of. What Titration Curve Of Glycine.
From chempedia.info
Glycine titration curve Big Chemical Encyclopedia What Titration Curve Of Glycine The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. Below is a typical curve for the titration of glycine with naoh. These curves allow the prediction of protonation states, charges, and isoelectric points. Titration curves of amino acids are very useful for. What Titration Curve Of Glycine.
From www.researchgate.net
1 pI of Glycine with Titration curve Download Scientific Diagram What Titration Curve Of Glycine The titration curve for glycine looks like the titration curve for a weak diprotic acid. Undergraduate biochemistry students should have great familiarity with titration curves. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. These curves allow the prediction of protonation states, charges, and isoelectric points. The isoelectric points range. What Titration Curve Of Glycine.
From www.youtube.com
Titration curve of glycine YouTube What Titration Curve Of Glycine These curves allow the prediction of protonation states, charges, and isoelectric points. Undergraduate biochemistry students should have great familiarity with titration curves. As you can see in the example for glycine shown below, a simple. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of. What Titration Curve Of Glycine.
From ar.inspiredpencil.com
Glycine Titration Curve What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. The isoelectric points range from 5.5 to 6.2. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. Titration curves of amino acids are very useful for identification. Undergraduate biochemistry. What Titration Curve Of Glycine.
From www3.nd.edu
Titration curve of glycine What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. In order to understand this. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. The isoelectric points range from 5.5 to 6.2. The titration curve for glycine looks like. What Titration Curve Of Glycine.
From www.slideserve.com
PPT Lecture 3 Amino Acids PowerPoint Presentation, free download What Titration Curve Of Glycine The titration curve for glycine looks like the titration curve for a weak diprotic acid. As you can see in the example for glycine shown below, a simple. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. The isoelectric points range from 5.5 to 6.2. Undergraduate biochemistry students should have. What Titration Curve Of Glycine.
From www.slideserve.com
PPT Amino Acids PowerPoint Presentation, free download ID9502455 What Titration Curve Of Glycine The isoelectric points range from 5.5 to 6.2. Below is a typical curve for the titration of glycine with naoh. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. The titration curve of glycine provides vital information about its basic or acidic. What Titration Curve Of Glycine.
From www.youtube.com
Titration Curve of Glycine YouTube What Titration Curve Of Glycine These curves allow the prediction of protonation states, charges, and isoelectric points. The titration curve for glycine looks like the titration curve for a weak diprotic acid. The results of these equations and the creation of a titration curve will signify that glycine is a zwitterion or a dipolar ion instead of a neutral. The figure below shows what happens. What Titration Curve Of Glycine.
From ar.inspiredpencil.com
Glycine Titration Curve What Titration Curve Of Glycine Below is a typical curve for the titration of glycine with naoh. The figure below shows what happens to the ph of an acidic solution of glycine when this amino acid is titrated with a strong base, such as naoh. In order to understand this. Titration curves of amino acids are very useful for identification. Undergraduate biochemistry students should have. What Titration Curve Of Glycine.
From www.studypool.com
SOLUTION Titration curve of glycine handout complete Studypool What Titration Curve Of Glycine As you can see in the example for glycine shown below, a simple. The isoelectric points range from 5.5 to 6.2. The titration curve of glycine provides vital information about its basic or acidic properties, its pka, and isoelectric point. The titration curve for glycine looks like the titration curve for a weak diprotic acid. These curves allow the prediction. What Titration Curve Of Glycine.