Boiling Point Of The Table Salt . The boiling point of water increases when salt is dissolved in it. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. It's true that part of why salt dissolves well in. This phenomenon is known as boiling point. Its melting point is 801°c, while its boiling point is 1,413°c. The oxidation state of an atom is. If your concentrations of salt are. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will. That is 58 grams per half degree celsius for each liter or kilogram of. The sodium and chlorine atoms are present in. Any solute in water raises the boiling point, so long as the solute stays in the liquid water.
from slidetodoc.com
Table salt is an ionic compound, which breaks into its component ions or dissociates in water. This phenomenon is known as boiling point. That is 58 grams per half degree celsius for each liter or kilogram of. The temperature needed to boil will. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. The oxidation state of an atom is. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. If your concentrations of salt are. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c.
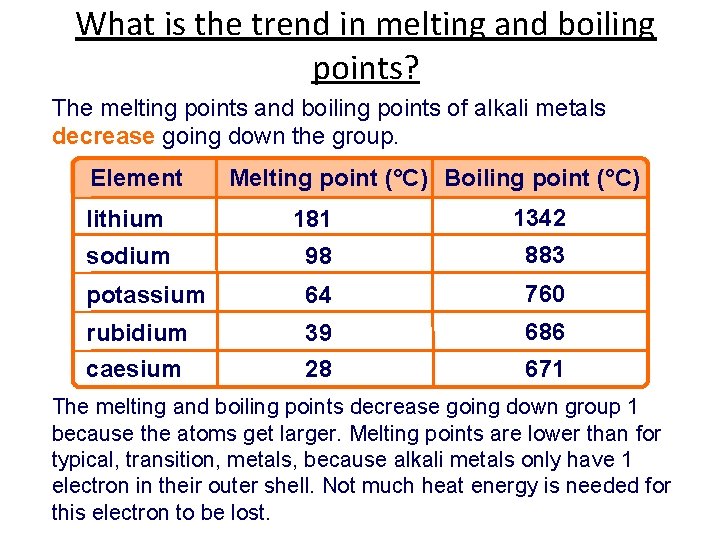
THE ALKALI METALS Canvas lesson 04 The Alkali
Boiling Point Of The Table Salt That is 58 grams per half degree celsius for each liter or kilogram of. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. Its melting point is 801°c, while its boiling point is 1,413°c. This phenomenon is known as boiling point. The oxidation state of an atom is. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. That is 58 grams per half degree celsius for each liter or kilogram of. The temperature needed to boil will. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. It's true that part of why salt dissolves well in. The sodium and chlorine atoms are present in. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The boiling point of water increases when salt is dissolved in it. If your concentrations of salt are.
From studylib.net
Periodic Table Trends Melting Point and Boiling Point Boiling Point Of The Table Salt If your concentrations of salt are. The boiling point of water increases when salt is dissolved in it. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. The sodium and chlorine atoms are present in.. Boiling Point Of The Table Salt.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Alcohols Boiling Point Of The Table Salt The sodium and chlorine atoms are present in. If your concentrations of salt are. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Its melting point is 801°c, while its boiling point is 1,413°c. This phenomenon is known as boiling point. The oxidation state of an atom. Boiling Point Of The Table Salt.
From klahkiyli.blob.core.windows.net
What Is Boiling Point Of Salt Water at Tracy Mendoza blog Boiling Point Of The Table Salt Its melting point is 801°c, while its boiling point is 1,413°c. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The oxidation state of an atom is. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by. Boiling Point Of The Table Salt.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Of The Table Salt Table salt is an ionic compound, which breaks into its component ions or dissociates in water. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. The sodium and chlorine atoms are present in. The boiling point is raised by 0.5 degrees celsius for. Boiling Point Of The Table Salt.
From mavink.com
Melting And Boiling Point Chart Boiling Point Of The Table Salt The sodium and chlorine atoms are present in. The oxidation state of an atom is. Its melting point is 801°c, while its boiling point is 1,413°c. The boiling point of water increases when salt is dissolved in it. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Any solute in water raises the. Boiling Point Of The Table Salt.
From userenginelegumes.z14.web.core.windows.net
The Normal Boiling Point Boiling Point Of The Table Salt Table salt is an ionic compound, which breaks into its component ions or dissociates in water. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. This phenomenon is known as boiling point. The temperature needed to boil will. It's true that part of. Boiling Point Of The Table Salt.
From www.thoughtco.com
Why Adding Salt to Water Increases the Boiling Point Boiling Point Of The Table Salt The temperature needed to boil will. This phenomenon is known as boiling point. If your concentrations of salt are. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The oxidation state of an atom is. In reality, you would need to add 230 grams of table salt. Boiling Point Of The Table Salt.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Boiling Point Of The Table Salt The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. The sodium and chlorine atoms are present in. That is 58 grams per half degree celsius for each liter or. Boiling Point Of The Table Salt.
From www.youtube.com
Boiling Table Salt (Sodium Chloride) Into a Gas! [Full HD] YouTube Boiling Point Of The Table Salt The oxidation state of an atom is. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The sodium and chlorine atoms are present in. That is 58 grams per half degree celsius for each liter or kilogram of. In reality, you would need to add 230 grams. Boiling Point Of The Table Salt.
From storyteller.travel
Does Salt Make Water Boil Faster? 5 Tips to Boil it Faster Boiling Point Of The Table Salt The temperature needed to boil will. The boiling point of water increases when salt is dissolved in it. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Its melting point is 801°c, while its boiling point is 1,413°c. The oxidation state of an atom is. The boiling point is raised by 0.5 degrees. Boiling Point Of The Table Salt.
From ceghryvh.blob.core.windows.net
What Is Boiling Point Sea Level at Edwin Acosta blog Boiling Point Of The Table Salt Table salt is an ionic compound, which breaks into its component ions or dissociates in water. That is 58 grams per half degree celsius for each liter or kilogram of. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. Its melting point is. Boiling Point Of The Table Salt.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Boiling Point Of The Table Salt If your concentrations of salt are. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. It's true that part of why salt dissolves well in. That is 58 grams per half degree celsius for each liter or kilogram of. If you add salt to water, you raise the boiling point, or the temperature. Boiling Point Of The Table Salt.
From slidetodoc.com
THE ALKALI METALS Canvas lesson 04 The Alkali Boiling Point Of The Table Salt This phenomenon is known as boiling point. If your concentrations of salt are. The oxidation state of an atom is. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling. Boiling Point Of The Table Salt.
From www.researchgate.net
Melting and boiling points of select salt compounds Download Table Boiling Point Of The Table Salt It's true that part of why salt dissolves well in. This phenomenon is known as boiling point. If your concentrations of salt are. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. The oxidation state. Boiling Point Of The Table Salt.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Boiling Point Of The Table Salt The sodium and chlorine atoms are present in. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. The boiling point of water increases when salt is dissolved in it. The oxidation state of an atom is. The temperature needed to boil will. This. Boiling Point Of The Table Salt.
From loeaanczy.blob.core.windows.net
Chlorine Boiling Point And Melting Point at Jane Gibbs blog Boiling Point Of The Table Salt The sodium and chlorine atoms are present in. Its melting point is 801°c, while its boiling point is 1,413°c. It's true that part of why salt dissolves well in. If your concentrations of salt are. That is 58 grams per half degree celsius for each liter or kilogram of. This phenomenon is known as boiling point. The temperature needed to. Boiling Point Of The Table Salt.
From www.studocu.com
Determination of boiling point organic Determination of boiling point Boiling Point Of The Table Salt Its melting point is 801°c, while its boiling point is 1,413°c. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point of water increases when salt is dissolved in it. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. The temperature needed. Boiling Point Of The Table Salt.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Boiling Point Of The Table Salt Its melting point is 801°c, while its boiling point is 1,413°c. It's true that part of why salt dissolves well in. That is 58 grams per half degree celsius for each liter or kilogram of. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2°. Boiling Point Of The Table Salt.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint Boiling Point Of The Table Salt Table salt is an ionic compound, which breaks into its component ions or dissociates in water. The oxidation state of an atom is. The sodium and chlorine atoms are present in. The boiling point of water increases when salt is dissolved in it. It's true that part of why salt dissolves well in. Its melting point is 801°c, while its. Boiling Point Of The Table Salt.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free Boiling Point Of The Table Salt The temperature needed to boil will. The boiling point of water increases when salt is dissolved in it. This phenomenon is known as boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The oxidation state of an atom is. Table salt is an ionic compound, which breaks into its. Boiling Point Of The Table Salt.
From www.chemix-chemistry-software.com
Melting and boiling points table of the elements Boiling Point Of The Table Salt If your concentrations of salt are. Its melting point is 801°c, while its boiling point is 1,413°c. This phenomenon is known as boiling point. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. If you. Boiling Point Of The Table Salt.
From studylib.net
Salt affect the freezing and boiling points of waterw Boiling Point Of The Table Salt This phenomenon is known as boiling point. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Its melting point is 801°c, while its boiling point is 1,413°c. In reality, you would need to add 230 grams of table salt to a liter of water just to raise. Boiling Point Of The Table Salt.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog Boiling Point Of The Table Salt Table salt is an ionic compound, which breaks into its component ions or dissociates in water. The sodium and chlorine atoms are present in. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The temperature needed to boil will. The oxidation state of an atom is. If. Boiling Point Of The Table Salt.
From www.youtube.com
4 2 6 aldehydes and ketones boiling point and solubility YouTube Boiling Point Of The Table Salt The oxidation state of an atom is. Its melting point is 801°c, while its boiling point is 1,413°c. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. In reality, you would need to add 230. Boiling Point Of The Table Salt.
From www.slideserve.com
PPT Ionic Bonds and Properties of Ionic Compounds PowerPoint Boiling Point Of The Table Salt In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. The sodium and chlorine atoms are present in. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The temperature needed to. Boiling Point Of The Table Salt.
From klahkiyli.blob.core.windows.net
What Is Boiling Point Of Salt Water at Tracy Mendoza blog Boiling Point Of The Table Salt That is 58 grams per half degree celsius for each liter or kilogram of. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The sodium and chlorine atoms are present in. The temperature needed to boil will. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams. Boiling Point Of The Table Salt.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Boiling Point Of The Table Salt If your concentrations of salt are. The oxidation state of an atom is. If you add salt to water, you raise the boiling point, or the temperature at which water boils. This phenomenon is known as boiling point. The temperature needed to boil will. Any solute in water raises the boiling point, so long as the solute stays in the. Boiling Point Of The Table Salt.
From www.youtube.com
melting and boiling points of elements YouTube Boiling Point Of The Table Salt If your concentrations of salt are. It's true that part of why salt dissolves well in. That is 58 grams per half degree celsius for each liter or kilogram of. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. If you add salt to water, you raise the boiling point, or the temperature. Boiling Point Of The Table Salt.
From www.researchgate.net
Melting and boiling points for the elements of the periodic table (data Boiling Point Of The Table Salt It's true that part of why salt dissolves well in. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling point by 2° c. That is 58 grams per half degree celsius for each liter or kilogram of. Any solute in water raises the boiling point, so long. Boiling Point Of The Table Salt.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube Boiling Point Of The Table Salt If your concentrations of salt are. The boiling point of water increases when salt is dissolved in it. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. This phenomenon is known. Boiling Point Of The Table Salt.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube Boiling Point Of The Table Salt The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. This phenomenon is known as boiling point. The boiling point of water increases when salt is dissolved in it. It's true that part of why salt dissolves well in. The temperature needed to boil will. If your concentrations. Boiling Point Of The Table Salt.
From pubchem.ncbi.nlm.nih.gov
Boiling Point Periodic Table of Elements PubChem Boiling Point Of The Table Salt This phenomenon is known as boiling point. The temperature needed to boil will. That is 58 grams per half degree celsius for each liter or kilogram of. The sodium and chlorine atoms are present in. If your concentrations of salt are. Any solute in water raises the boiling point, so long as the solute stays in the liquid water. It's. Boiling Point Of The Table Salt.
From webapi.bu.edu
🌱 How does salt affect boiling point. To investigate the effect of the Boiling Point Of The Table Salt The temperature needed to boil will. Table salt is an ionic compound, which breaks into its component ions or dissociates in water. That is 58 grams per half degree celsius for each liter or kilogram of. It's true that part of why salt dissolves well in. The boiling point of water increases when salt is dissolved in it. The sodium. Boiling Point Of The Table Salt.
From www.slideserve.com
PPT CHAPTER 8 INTRODUCTION TO ORGANIC CHEMISTRY PowerPoint Boiling Point Of The Table Salt Its melting point is 801°c, while its boiling point is 1,413°c. If your concentrations of salt are. That is 58 grams per half degree celsius for each liter or kilogram of. The sodium and chlorine atoms are present in. It's true that part of why salt dissolves well in. The boiling point is raised by 0.5 degrees celsius for water. Boiling Point Of The Table Salt.
From byjus.com
what is the order of melting and boiling points in the 13^th group Boiling Point Of The Table Salt Its melting point is 801°c, while its boiling point is 1,413°c. This phenomenon is known as boiling point. That is 58 grams per half degree celsius for each liter or kilogram of. The temperature needed to boil will. In reality, you would need to add 230 grams of table salt to a liter of water just to raise the boiling. Boiling Point Of The Table Salt.